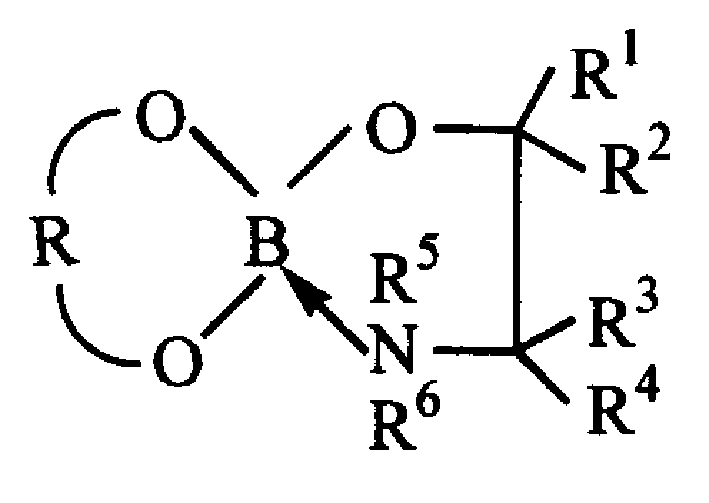
Chelating chiral boric acid ester and preparatio process and use thereof
A boronate, chiral technology, applied in the field of chiral compound preparation chemistry, to achieve the effects of strong oxidation resistance, high thermal stability and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of (R)-1,1-bi-2-naphtholboronic acid-(S)-(2-pyrrolidinyl)-1,1-diphenylmethyl ester: 2.86g (10mmol)(R)- 1,1′-bi-2-naphthol and 2.4mL (1.96g, 10.08mmol) triisopropyl borate were refluxed in toluene for 6 hours and then stoichiometric (S)-(2-pyrrolidinyl)- 1,1-Diphenylmethanol, continue to reflux for 6 hours, cool down, evaporate (80-90°C) toluene and isopropanol under reduced pressure with a water pump, and treat the residue with ethanol to obtain (R)-linked- 2-Naphtholboronic acid-(S)-(2-pyrrolidinyl)-1,1-diphenylmethyl ester.
Embodiment 2
[0018] The preparation of catechol boric acid (S)-proline anhydride: similar to Example 1, catechol and triisopropyl borate were refluxed in benzene and added stoichiometric (S)-proline after 6 hours to continue After reflux for 6 hours, an oily substance was generated and solidified after cooling. The solid was separated and crystallized from methanol to give 61% catecholboronic acid (S)-proline anhydride, m.p. 196-198°C.
Embodiment 3
[0020] Preparation of (S)-1,1'-bi-2-naphthol boronic acid-quinalidine anhydride: (S)-1,1'-bi-2-naphthol (2.86g, 10mmol) and 0.92M BH 3 THF (12ml, 11mmol) solution was reacted at room temperature in THF for 2 hours; the solvent and excess borane were evaporated under reduced pressure; anhydrous quinaldinic acid (1.75 grams, 10mmol) and benzene (40mL) were added, stirred at room temperature for 0.5 hours After that, it was refluxed for another 2 hours; (S)-bi-2-naphtholboronic acid-quinalidine anhydride was almost quantitatively obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com