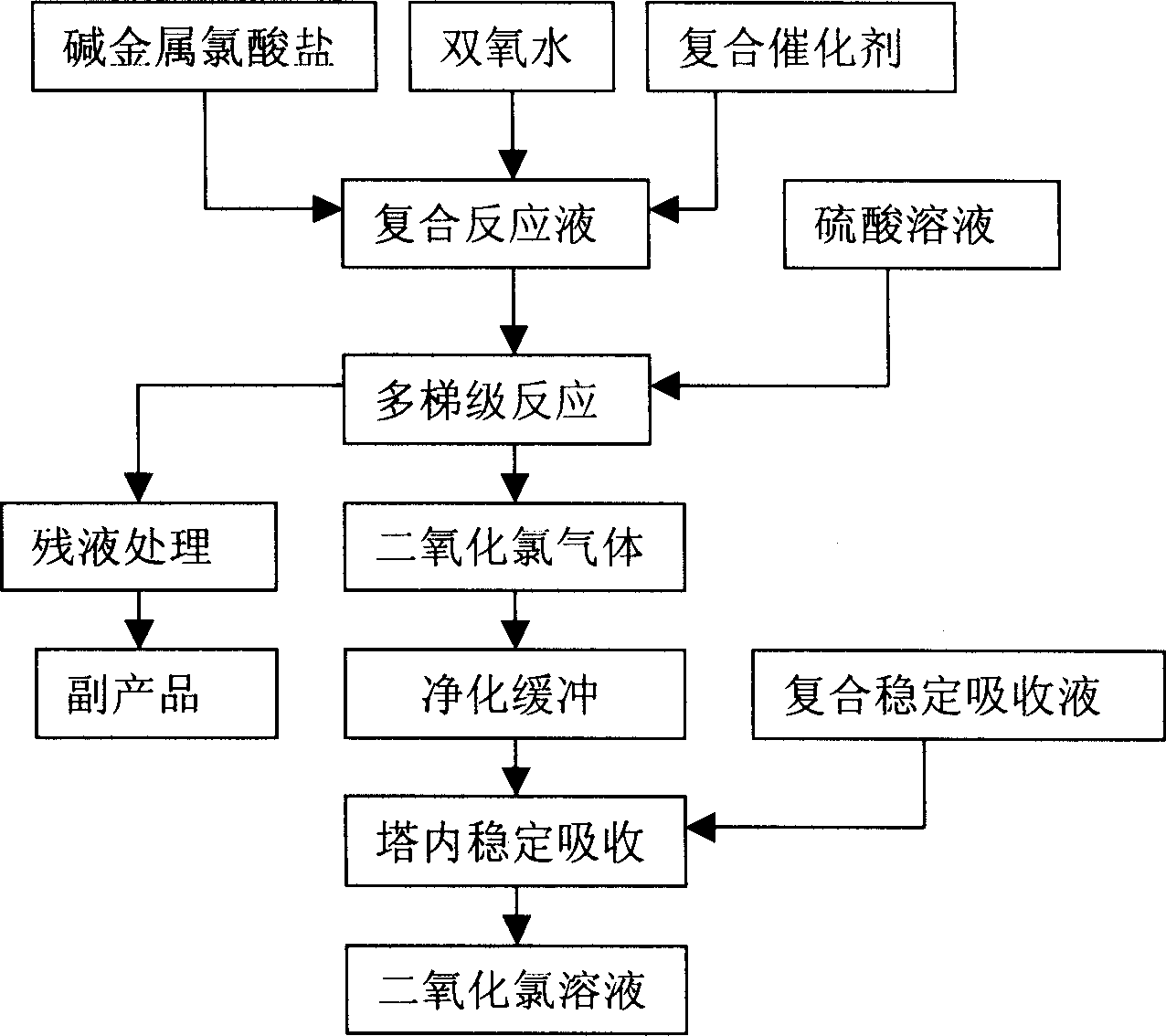
Production for stable chlorinedioxide solution
A chlorine dioxide and production method technology, applied in the direction of chlorine dioxide and the like, can solve the problems of difficulty in producing high-concentration, high-purity stable chlorine dioxide solution, difficult to accurately control process operation, difficult to control reaction process, etc. Continuous operation process, complete conversion of raw materials, and the effect of solving instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] project
Embodiment 2
[0027] Determine the product specification concentration as 4%. The weight ratio of composite catalyst sodium pyrophosphate, 2-hydroxyphosphonoacetic acid and urea is 1: 0.4: 0.6; the weight ratio of sodium chlorate, hydrogen peroxide, demineralized water and composite catalyst in the composite reaction solution is 1: 0.65: 0.76: 0.008; add composite stable absorption liquid to the combined absorption tower, the proportion is sodium carbonate 4%; sodium hydroxide 1%; hydrogen peroxide 8%; deionized water 87%, hydrogen peroxide concentration 30% , adjust the compound reaction liquid flow rate to 22L / h, the sulfuric acid solution flow rate to 26L / h, control the reaction negative pressure of the multi-step reactor at 0.04-0.42Mpa, and the reaction temperature at 32-35°C. After running for 15 hours, discharge 3.07 tons of stable chlorine dioxide aqueous solution, calculate raw material consumption cost to be 703 yuan / ton product, total yield 88.2%, measure chlorine dioxide purity ...
Embodiment 3
[0029] Determine the product specification concentration as 8%. The weight ratio of composite catalyst sodium pyrophosphate, 2-hydroxyphosphonoacetic acid and urea is 1: 0.5: 0.1; the weight ratio of sodium chlorate, hydrogen peroxide, demineralized water and composite catalyst in the composite reaction solution is 1: 0.65 : 0.8: 0.008; In the combined absorption tower, add composite stable absorption liquid, proportioning is sodium carbonate 5%; Sodium hydroxide 4.5%; Hydrogen peroxide 16%; Deionized water 74.5%, adjust composite reaction liquid flow rate to be 27.5% L / h, the sulfuric acid solution flow rate is 18.5L / h; the pure water flow rate: 14L / h; the reaction negative pressure of the multi-step reactor is controlled at 0.04-0.42Mpa, and the reaction temperature is 32-35°C. After running for 8 hours, discharge 1.08 tons of stable chlorine dioxide aqueous solution, calculate raw material consumption cost to be 1448 yuan / ton product, total yield 87.9%, measure chlorine dio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com