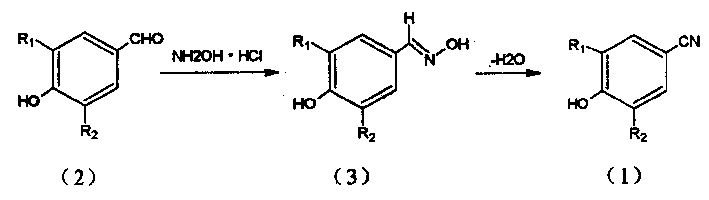
Method of preparing p-cyanophenol like compound
A technology of p-cyanophenol and compounds, which is applied in the field of p-cyano-substituted phenol compounds, can solve the problems of many side reactions, increase the difficulty and cost of production, and pollute the environment, so as to reduce costs and expenses, and solve pollution and hazards , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1: the preparation of 2-chloro-6-methyl p-cyanophenol
[0012] 154.5g (1.08mol) of 2-chloro-6-methylphenol was dissolved in 1000ml of trifluoroacetic acid, and 152g (1.08mol) of hexamethylenetetramine was added in batches at room temperature, and an exothermic reaction occurred. , then add 300ml trifluoroacetic acid to facilitate stirring. The float was heated to reflux overnight and the reaction mixture was cooled. Excessive trifluoroacetic acid was recovered by distillation under reduced pressure, and the mixture was poured into 3000ml of CH 2 Cl 2 , the organic layer was washed with water, and then each time with 500ml containing 10% K 2 CO 3 The solution was washed three times, the aqueous extract was acidified with concentrated hydrochloric acid, the precipitate was collected, and recrystallized with ethyl acetate to obtain 115 g of the corresponding p-hydroxybenzaldehyde compound (II), yield 62%, mp: 114-116°C.
[0013] Compound (II) was dissol...
Embodiment 2
[0015] Embodiment 2: the synthesis of 3.5-dimethyl p-hydroxybenzaldoxime
[0016] Add 15g (0.1mol) of 3,5-dimethyl p-hydroxybenzaldehyde (V), add NaOH solution (8g+50ml water), 7g of hydroxylamine hydrochloride, reflux the mixture for 4 hours, cool, and use 30% acetic acid under ice bath Adjust the pH to about 6, filter, wash with water, and recrystallize with chloroform to obtain 3,5-dimethyl-p-hydroxybenzaldehyde oxime (VI), 14.8g, yield 90.2%, mp: 83-85°C.
[0017] Put the obtained solid (VI) into the bottle, add 150ml of toluene, then add 0.5ml of concentrated sulfuric acid, stir and reflux and separate the water produced by the reaction, react for 2 hours, pour the saturated sodium phosphate solution into the reactant and mix, separate the water layer, concentrated a part, cooled and crystallized, and filtered to obtain 12 g of the target compound (VII) in light yellow needle-like crystals, 3,5-dimethyl-p-hydroxybenzonitrile, yield 90.9%, mp: 121-122C.
[0018] H-NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com