DNA vaccine pVPH for SARS virus
A technology of SARS virus and DNA vaccine, which is applied in the field of medical bioengineering, to achieve the effect of simple preparation process, strong effect and strong prevention of virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation of SARS virus DNA vaccine pVPH
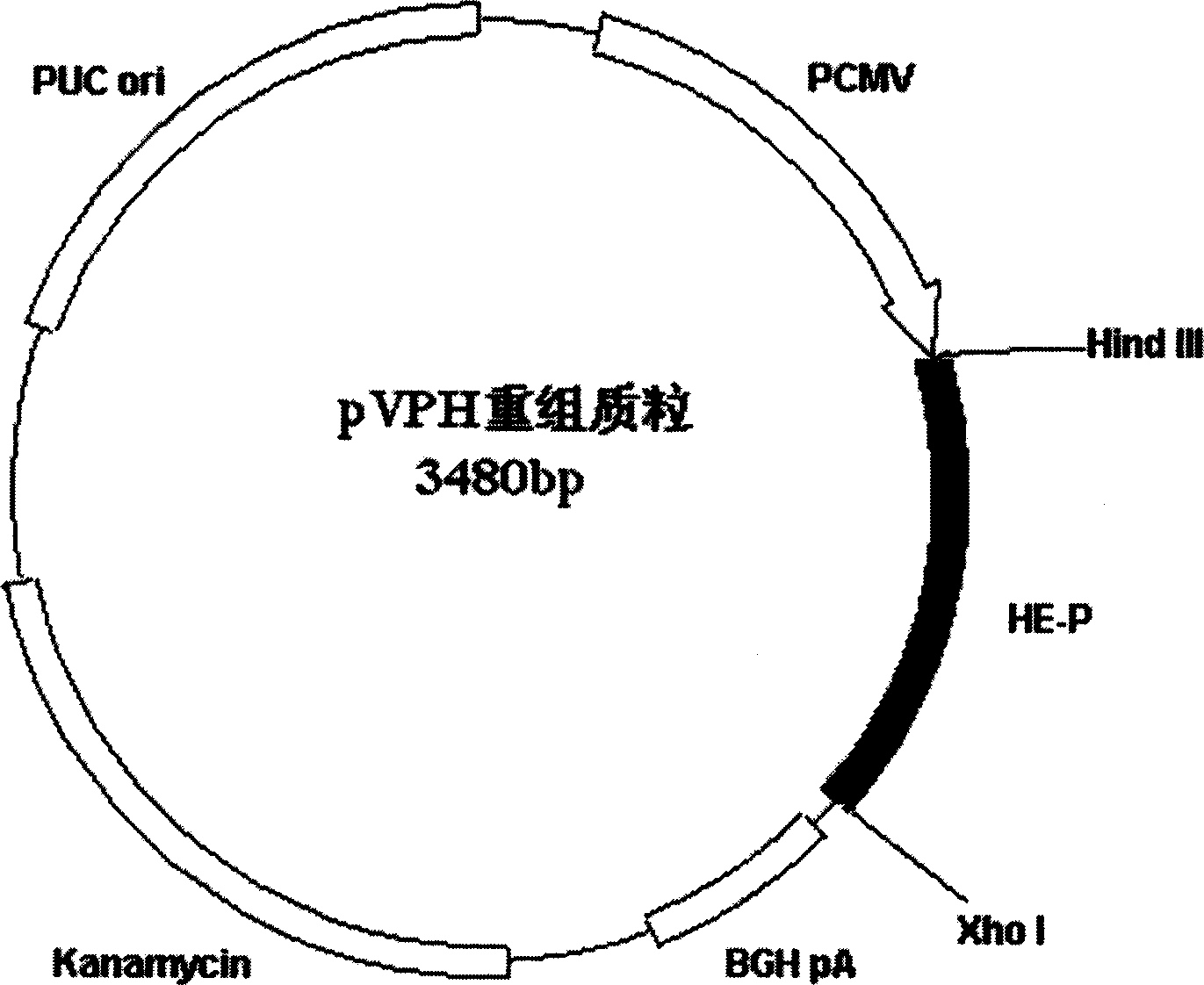
[0028] DNA vaccine pVPH structure see figure 1 , the construction process is shown in Figure 2.
[0029] 1. Synthetic HE-PDNA sequence (structure as described above), its 5' end contains Hind III restriction site 3' end contains Xho I restriction site
[0030] 2. Construct the recombinant plasmid of pVPH:
[0031] The HE-P DNA fragment was double digested with restriction endonucleases Hind III and Xho I. The reaction system is: HE-P DNA fragment 5μl (200ng / μl); 10×M Buffer (100mM Tris-Hcl pH7.5; 100mM MgCl 2 ; 10mM Dithionthreitol; 500mM NaCl) 2μl; Hind III enzyme (10U / μl) 1μl; Xho I enzyme (12U / μl) 1μl; 2 O to a total volume of 20 μl. Digest in a warm bath at 37°C for 2 hours. Afterwards, the digested samples were subjected to 1% low-melting point agarose gel electrophoresis, and the gel was cut to recover M-P fragments. At the same time, the pVAX1 vector was digested with Hind III and Xho I. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com