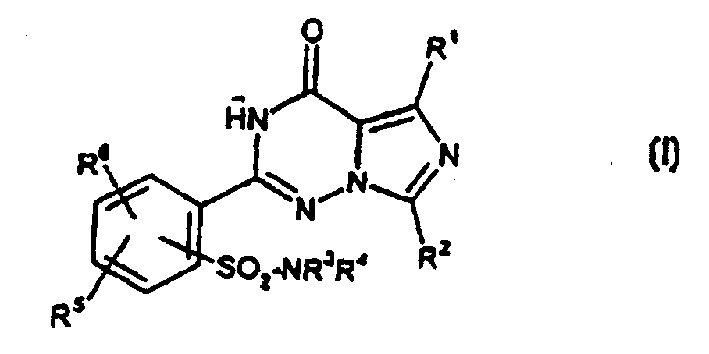
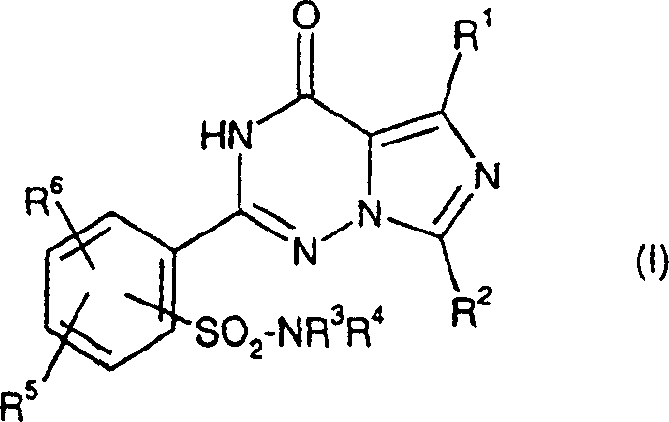
2-phenyl-substituted imidazo-triazone used as phosphodiesterase inhibitor
A kind of phenyl substitution, imidazo technology, is applied in the application field of phosphodiesterase inhibitor, can solve the problem such as not requiring protection sulfonamido
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0467] 2-Butyrylaminopropionic acid
[0468]
[0469] 22.27 g (250 mmol) of D, L-alanine and 55.66 g (550 mmol) of triethylamine were dissolved in 250 ml of dichloromethane, and the solution was cooled to 0°C. 59.75 g (550 mmol) of trimethylsilyl chloride were added dropwise, and the solution was stirred at room temperature for 1 hour and at 40° C. for 1 hour. After cooling to -10°C, 26.64 g (250 mmol) of butyryl chloride was added dropwise, and the resulting mixture was stirred at -10°C for 2 hours and at room temperature for 1 hour.
[0470] Under ice-cooling, 125 ml of water were added dropwise, and the reaction mixture was stirred at room temperature for 15 minutes. The aqueous phase was evaporated to dryness, the residue was triturated with acetone and the mother liquor was filtered. The solvent was removed and the residue was chromatographed. The resulting product was dissolved in 3 N aqueous sodium hydroxide solution, and the resulting solution was evaporated to d...
Embodiment 2A
[0474] 2-Butyrylaminobutyric acid
[0475]
[0476] 25.78 g of 2-aminobutyric acid (250 mmol) and 55.66 g (550 mmol) of triethylamine were dissolved in 250 ml of dichloromethane, and the solution was cooled to 0°C. 59.75 g (550 mmol) of trimethylsilyl chloride were added dropwise, and the solution was stirred at room temperature for 1 hour and at 40° C. for 1 hour. After cooling to -10°C, 26.64 g (250 mmol) of butyryl chloride was added dropwise, and the resulting mixture was stirred at -10°C for 2 hours and at room temperature for 1 hour.
[0477] Under ice-cooling, 125 ml of water were added dropwise, and the reaction mixture was stirred at room temperature for 15 minutes. The organic phase was mixed with aqueous sodium hydroxide solution, and the organic solvent was removed under reduced pressure. After acidification, the precipitated solid was stirred once with water and twice with petroleum ether, and dried under reduced pressure at 45°C. 29.1 g (67%) of a colorless...
Embodiment 3A
[0481] 2-Ethoxybenzonitrile
[0482]
[0483] 25 g (210 mmol) of 2-hydroxybenzonitrile were refluxed overnight in 500 ml of acetone with 87 g of potassium carbonate and 34.3 g (314.8 mmol) of ethyl bromide. The solid was filtered off, the solvent was removed under reduced pressure, and the residue was distilled under reduced pressure. 30.0 g (97%) of a colorless liquid were obtained.
[0484] 200MHz 1 H-NMR (DMSO-d6): 1.48, t, 3H; 4.15, quart., 2H; 6.99, dt, 2H; 7.51, dt,
[0485] 2H.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com