Novel high-purity silybin meglumine medicine preparation as well as preparation method and application thereof
A technology of silibinin and pharmaceutical preparations, which is applied in the field of pharmaceutical preparation technology and pharmacology, can solve the problems of low bioavailability, low absolute value, low stability, etc., and achieve high bioavailability, short half-life, and fast absorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
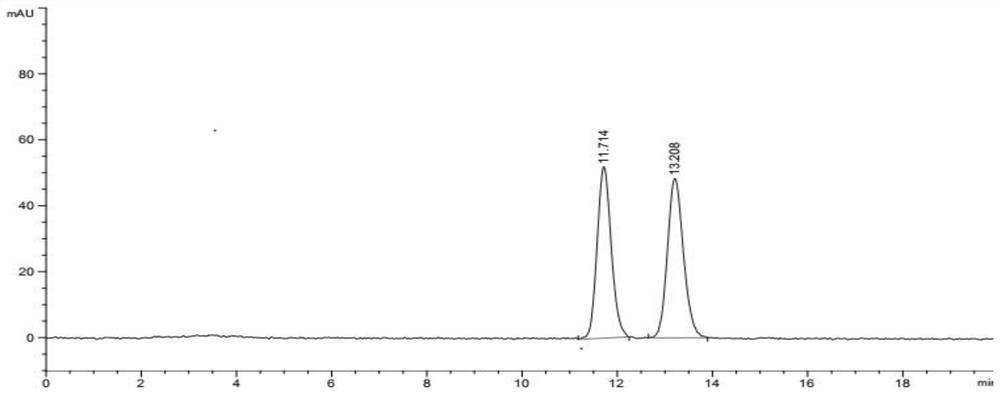
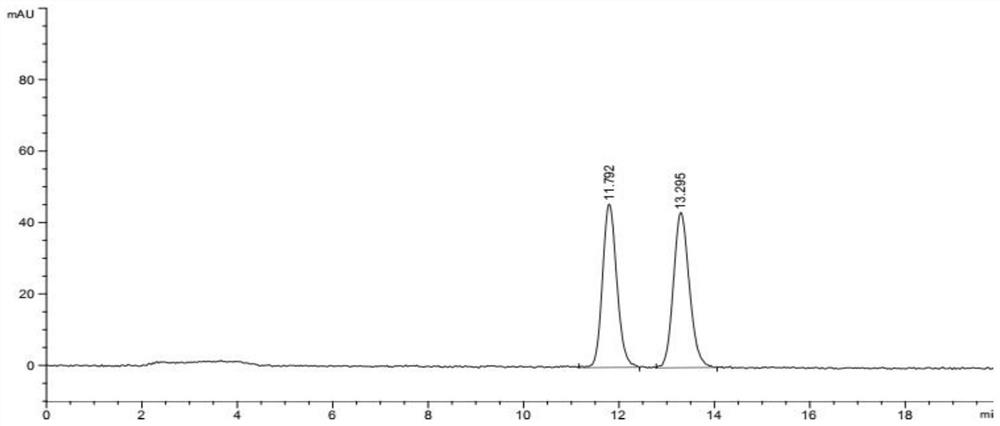
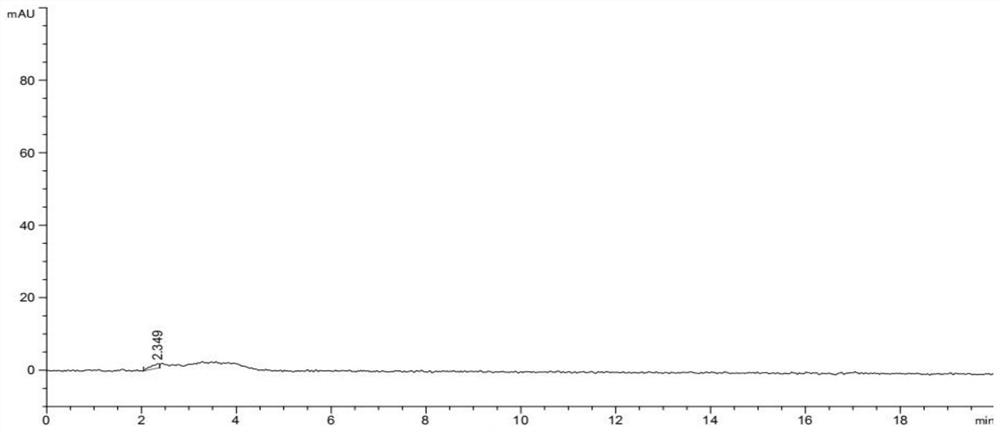
Image
Examples
Embodiment 1
[0114] Embodiment 1 Preparation of silybin meglumine sustained-release pellet capsules
[0115] Prescription specification: silybin meglumine 150.0mg / capsule, composed of:
[0116] (1) Prescription of sustained-release pellet core:
[0117]
[0118]
[0119] (2) Prescription of sustained-release coating liquid
[0120]
[0121] (3) Process
[0122] ①, raw materials pretreatment
[0123] The raw and auxiliary materials were respectively passed through 80 mesh sieves, and the prescription amount was weighed for use;
[0124] Dissolve PVPk30 with 60% ethanol to prepare a 5wt.% PVPK30 ethanol solution;
[0125] Take the slow-release 12h coating material (ethyl cellulose, dibutyl sebacate, PVPk30 and 60% ethanol) and add an appropriate amount of purified water to make a 12 wt.% suspension to fully swell;
[0126] ②, mixing and making soft materials
[0127] Weigh silibinin meglumine, microcrystalline cellulose, sucrose, and sodium chloride according to the prescripti...
Embodiment 2
[0143] Preparation of silibinin meglumine for injection
[0144] prescription:
[0145]
[0146]
[0147] Preparation:
[0148] 1. Preparation Take PVPc12, add 900ml of freshly prepared water for injection at 10-15°C and stir to dissolve, add high-purity silibinin meglumine and stir to dissolve, add freshly prepared water for injection at 10-15°C to 1000ml, and use 0.2μm cross-flow fine filtration, first circularly filtered for 3min and then filtered to collect the filtrate.
[0149] 2. Dispense it into 7ml borosilicate bottles, 1.0ml (25mg) per bottle, and suspend the stopper.
[0150] 3. Freeze-drying Immediately place the vials in a freeze dryer that has been pre-cooled to -50°C, freeze for 2 hours, start drying for 20 hours, then rise to 25°C for 2 hours, heat up to 35°C for 2 hours, and press the stopper.
[0151] 4. Packing with lids Take out the freeze-dried products with pressed plugs, tie aluminum-plastic composite lids, label, pack, and store them.
[0152]...
Embodiment 3
[0155] Pharmacodynamic study on the prevention and treatment of acute liver failure with silybin meglumine (SM) for injection (iv)
[0156] experimental method:
[0157] (1) Experimental animals
[0158] The experimental animals were C57BL / 6J male mice, weighing 22-25 g, 8 weeks old, SPF grade, purchased from the Comparative Medical Center of Yangzhou University, which met the quality standards of ordinary experimental animals. License number SYXK (Su) 2018-0019. Mice were reared in separate cages, with free access to water and food, at room temperature of 23 to 25°C and relative humidity of 40 to 80%.
[0159] (2) Animal grouping and drug intervention
[0160] The C57BL / 6J mice were randomly divided into 6 groups, namely blank group, model group, SM 1.25 mg / kg, SM 2.5 mg / kg, SM 5 mg / kg, acetylcysteine injection group (NAC group 1.04 mg / kg). g / kg). The acute liver failure model was induced in mice by intraperitoneal injection of D-GalN (500mg / kg) combined with 10LPS (μg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com