Recombination human alkaline fiber forming cell growth factor gene and its nonfusion expression product, production method and application
A fibroblast, growth factor technology, applied in the field of genetic engineering applications, can solve the problems of non-expression, fast mRNA translation, slow mRNA translation, etc., and achieve the effects of high translation initiation efficiency, high expression, and improved expression rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of hbFGF gene modified in TIR region
[0057] Design upstream primers as follows:
[0058] F1: GAA GCT GCT AGT ATT ACG ACA CTG CCG GCT CTG CCG GAA GAT GGT
[0059] GGT AGC GGT GCT
[0060] F2: GAA GCT GCT GGT AGT ATT ACG ACG CTG CCG GCC CTG CCG GAA GAT
[0061] GGT GGT AGT GGT GCA
[0062] F3: GAA GCT GCT GGT AGT ATT ACT ACA CTG CCG GCC CTG CCG GAA GAT GGT
[0063] GTT AGC GGT GCG
[0064] F4: GAA GCT GCT GGT AGT ATT ACT ACG CTG CCG GCT CTG CCG GAA GAT GGT
[0065] GGT AGT GGT GCG
[0066] F5: GAA GCT GCT GGT AGC ATT ACA ACT CTG CCG GCA CTG CCG GAA GAT
[0067] GGT GGT AGT GGT GCC
[0068] F6: GAA GCT GCT GGT AGT ATT ACA ACT CTG CCG GCA CTG CCG GAA GAT
[0069] GGT GGT AGT GGT GCA
[0070] F7: GAA GCT GCT GGT AGC ATT ACG ACC CTG CCG GCG CTG CCG GAA GAT
[0071] GGT GAT AGT GGT GCT
[0072] Design downstream primers as follows:
[0073] R: GACA TTA TCA GCT CTT AGC AGA CAT TGG
[0074] The base sequence in the box is th...
Embodiment 2
[0081] Example 2: Construction of pET-JN and digestion of recombinants
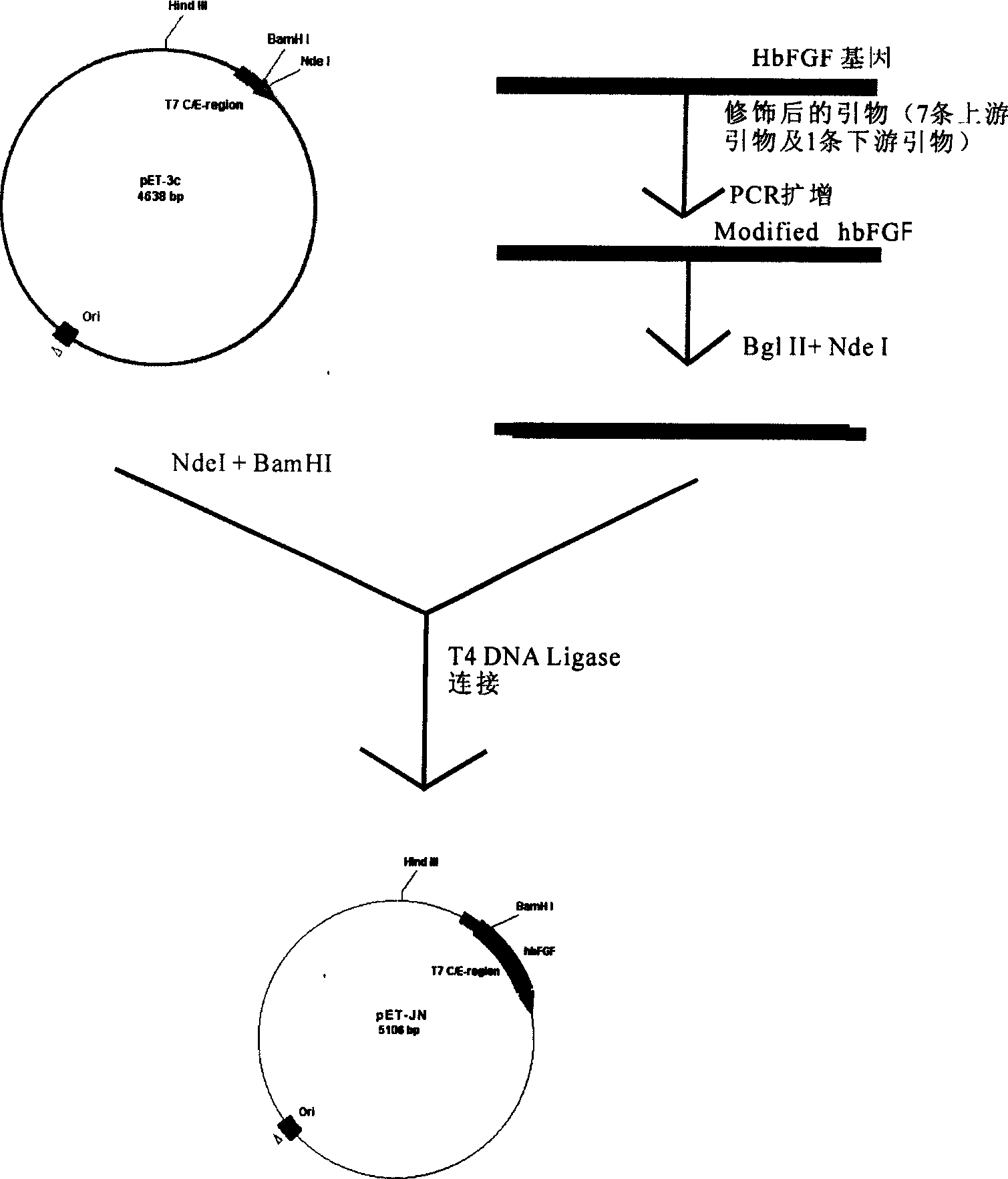
[0082] The digested and purified DNA fragments of vector plasmid DNA pET-3c / Nde I / BamH I and hbFGF / Nde I / Bgl II were ligated in a constant temperature water bath at 16°C for 30 minutes according to conventional methods, and when the ligation reaction was completed, , to transform DH5α competent cells, take 100 μL of the transformation solution and apply it to AMP-resistant LB plate, and culture it in a 37°C incubator for 12-16h. See the build process figure 1
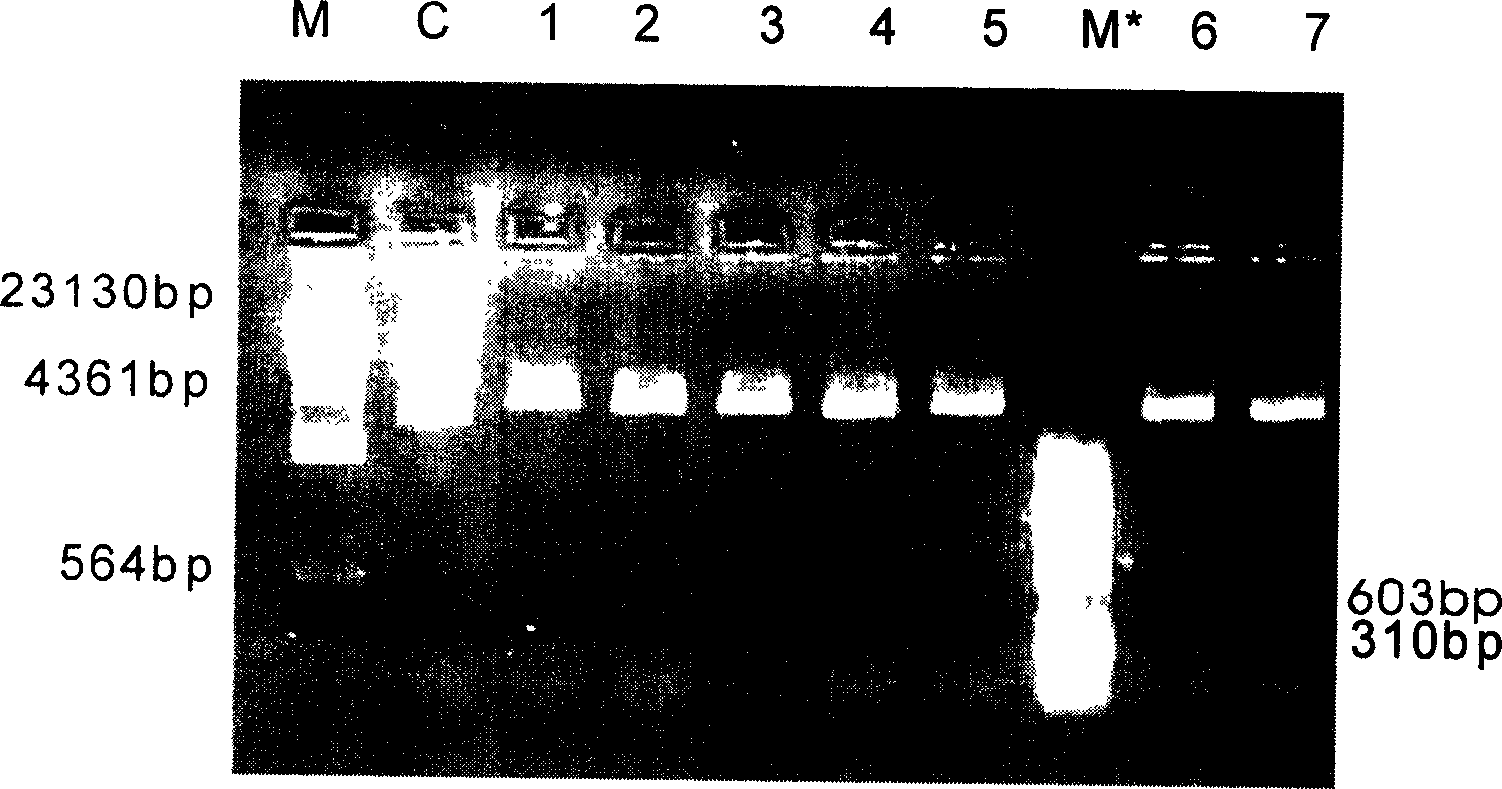
[0083] Use a sterile toothpick to pick 3-5 colonies grown on each resistant plate, inoculate them into test tubes of 5 mL liquid LB medium with 100 μg / mL AMP, and culture with shaking at 37°C and 200 rpm / min for 12-16 hours Afterwards, use the alkali method to extract a small amount of plasmids, extract the plasmids for BamH I+Nde I double enzyme digestion, identify by electrophoresis, and select recombinants.
[0084] It can be seen from the f...
Embodiment 3
[0085] Example 3: Expression analysis of non-fusion hbFGF protein
[0086] The DH5α cells containing the recombinant plasmid pET-JN series identified by enzyme digestion were amplified in 5 mL LB liquid medium containing 100 μg / ml AMP, the plasmid was extracted by the alkaline method, and transformed into BL21(DE3)pLysS cells. Spread on LB culture plates containing 100 μg / ml AMP and chloramphenicol (Chloromycetin, hereinafter referred to as CHL) double antibody for culture, and culture overnight at 37°C. Pick positive colonies and inoculate them into 50ml LB test tubes with 100μg / ml AMP and 100μg / ml CHL for expression test, culture at 37°C until OD 600 was 0.8, IPTG (isopropyl-β-D-thiogalactoside) was added for induction for 4 hours.
[0087] In order to analyze the more detailed situation of the obtained bacterial strain expressing non-fusion hbFGF, the obtained bacterial strain with expression was further expanded and cultured, and after induction, the bacterial cells were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com