Polymer-made-micel contained administration system through skin or mucosa
A drug delivery system and polymer glue technology, which are applied in the directions of drug devices, drug delivery, and pharmaceutical formulations, to achieve the effects of simple preparation method, easy realization, and promotion of transmission and absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 (preparation of polymer assembly micelles):
[0025] Dissolve 95mg of polyethylene glycol monomethyl ether-b-poly(D,L-lactic acid) block copolymer (block ratio 2000 / 1500) and 5mg of paclitaxel in 2mL of acetone, and heat to 60°C under nitrogen protection Evaporate for 2 hours to obtain the drug / copolymer solid mixture, dry it in vacuum at room temperature, preheat the drug / copolymer solid mixture in a constant temperature water bath at 60°C until it becomes a transparent gel, add 60°C double distilled water or phosphate buffer under stirring (PBS, pH 7.6) 10mL to form a transparent micellar solution, centrifuged, and freeze-dried to obtain paclitaxel-loaded polymer micelles (PMT) lyophilized powder. The drug loading of PMT measured by high pressure liquid phase method is 4.9%.
[0026] The same method was used to prepare PMTs with different drug loadings, and the results are listed in Table 1.
[0027] Theoretical drug loading
[0028] Note: The...
Embodiment 2
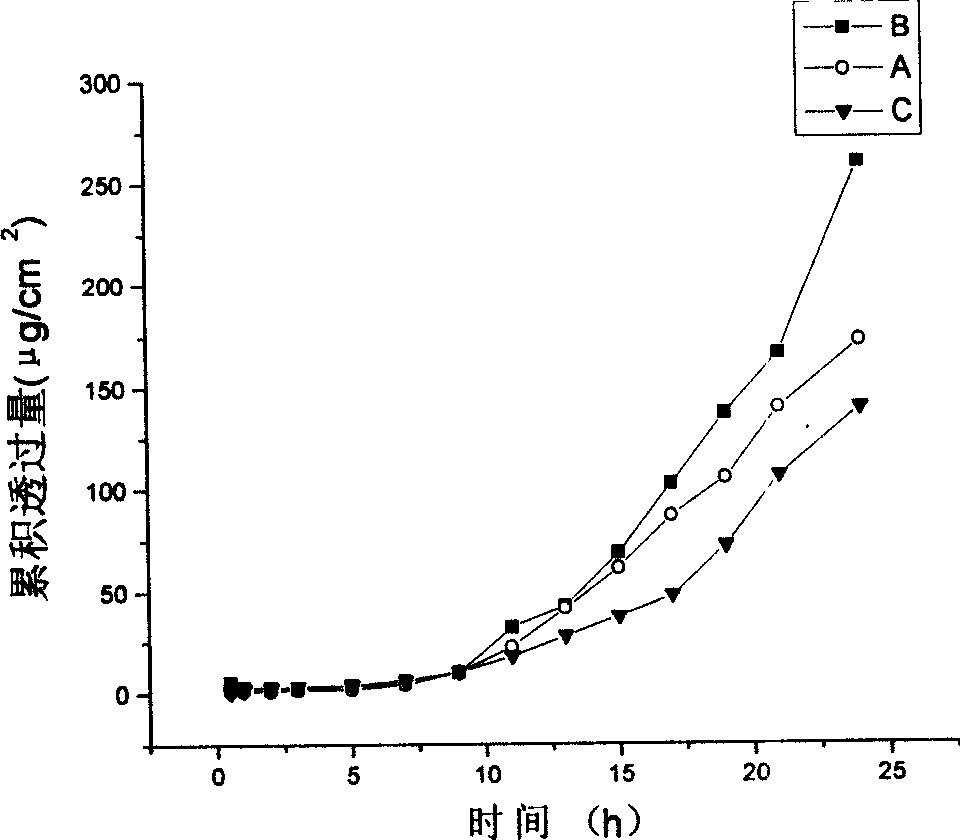
[0030] Take by weighing 0.1g of the PMT (drug loading 4.9%) lyophilized powder prepared by the method of Example 1, disperse it into 1mL distilled water to form the aqueous solution of PMT, and carry out the transdermal release experiment with the Franz diffusion cell in vitro, using a depilated large Rat abdominal skin (0.7cm 2 ), the receiving solution is a phosphate buffer solution with pH=7.4. In the same way, 1 mL of PMT aqueous solution containing the same amount of paclitaxel was prepared with PMT with a drug loading of 3.4% and 8.8%, and the percutaneous release experiment was carried out. The paclitaxel in the receiving solution was detected by HPLC. The result is as figure 1 shown.
[0031] During the transdermal release test of PMT aqueous solution, the receiving solution did not change significantly within the first 12 hours, and the receiving solution turned milky white after 24 hours. The transmission electron microscope photos of the PMT solution before relea...
Embodiment 3
[0036] The device and operation are the same as in Example 2, mix 4g of PEG3350 and 6g of PEG400 to form an ointment, and weigh 0.1g of PMT freeze-dried powder with a drug loading of 4.9% prepared in Example 1 and mix it with 1g of the ointment, and then apply it on Transdermal release experiments were performed on mouse skin. The result is as Figure 4 shown.
[0037] Figure 4 The result of curve A shows that PMT in ointment can also penetrate the skin, and the penetration amount is slightly lower than the aqueous solution of PMT.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com