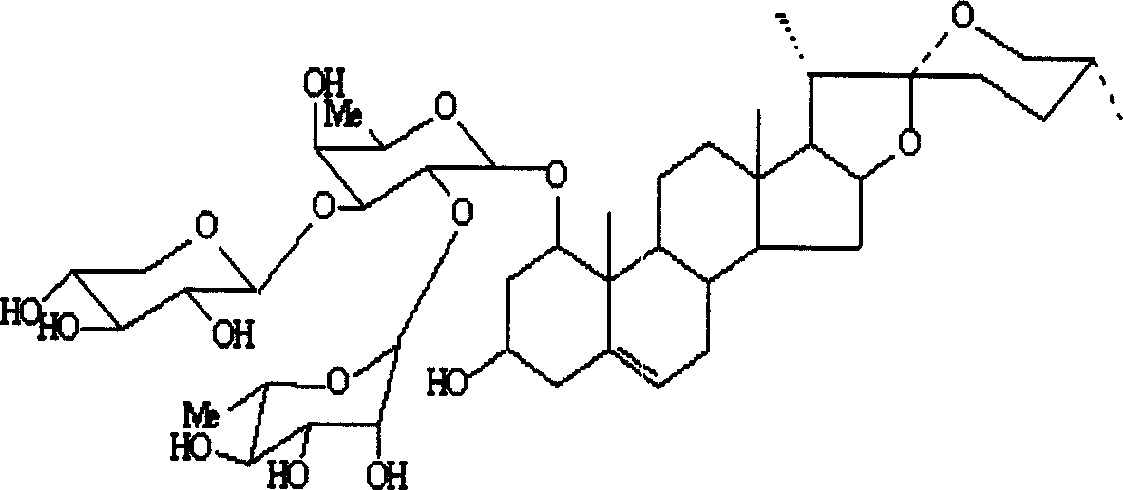
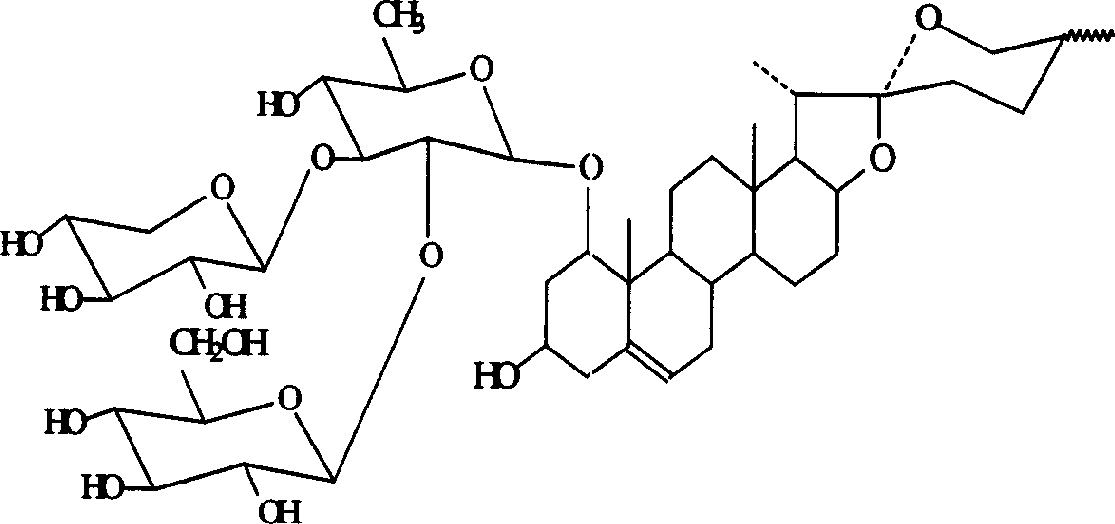
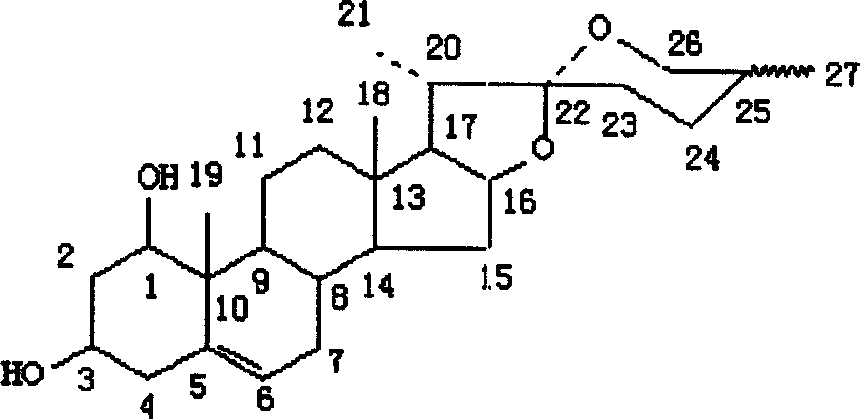
Rusco saponin element and application of its saponin in preparation of medicine preventing and treating embolus disease
A technology for ruscogenin and thrombotic diseases, applied in the field of application of ruscogenin and its saponins in the preparation of drugs for preventing and treating thrombotic diseases, capable of solving problems such as spontaneous bleeding and slow action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]Preparation method of ruscogenin and saponin thereof
[0038] 1. Total saponins
[0039] Take 2 kg of dried fibrous roots of Lithopriopogon japonicus (Short Ting Mountain or Hubei Radix Ophiopogon japonicus), soak for 1 hour, decoct for 3 times, each time for 1 hour, combine the decoction liquid, settle still, take the supernatant, pass it through macroporous resin , rinse with distilled water first, discard the eluate, then rinse with 40% ethanol, discard the eluate (no saponin components after testing), then rinse with 80% ethanol, concentrate the eluate and evaporate to dryness, that is The total saponins of Ophiopogon japonicus (the total saponins of Ophiopogon japonicus brevis is abbreviated as LM-ts).
[0040] 2. Leptosponin C (LM-C) and ruscogenin
[0041] 7.0kg of Ophiopogon japonicus fibrous roots, cold-soaked in 95% ethanol for 1 month, percolated with ethanol 3 times, combined, concentrated to an extract-like extract, extracted 3 times with ethyl acetate, co...
Embodiment 2
[0044] The preparation method of embodiment 2 ruscogenin and its saponin dosage form
[0045] 1. Capsule preparation method
[0046] prescription composition
[0047] CSO 50.0g Starch 47.5g Microcrystalline Cellulose 95.5g
[0048] Appropriate amount of 5% starch slurry talcum powder 2.0g made into 1000 capsules
[0049] Preparation Process
[0050] The total saponins of Ophiopogon japonicus are crushed into fine powder, mixed with auxiliary materials, made into granules, dried, packed into capsules, and packaged for quality inspection.
[0051] 2. Tablet preparation method
[0052] prescription composition
[0053] LM-C 20g Starch 400g Starch slurry (8%) Appropriate amount Magnesium stearate Appropriate amount
[0054] Made into 1000 pieces
[0055] Preparation Process
[0056] Take LM-C and mix it with starch, use 8% starch slurry as a binder to make a soft material, pass through a 12-14 mesh sieve and granulate, dry at 70-80°C, granulate, add magnesium stearate and ...
Embodiment 3
[0063] Example 3 Research on the antithrombotic activity of ruscogenin and its saponins
[0064] 1 Experimental materials
[0065] 1.1 Drugs and reagents
[0066] LM-ts, CSO, ophiopogonin D, LM-C, ruscogenin, all with a purity of more than 80%. Aspirin enteric-coated tablets (abbreviated as ASA, Nanjing Hengsheng Pharmaceutical Factory, batch number 030304); carrageenan (Carrageenan, TypeI, Sigma, C1013); warfarin sodium tablets (Warfarin, Shanghai Jiufu Pharmaceutical Co., Ltd., batch number: 030601).
[0067] 1.2 Experimental animals
[0068] Kunming mice, male, 18-22g and 25-30g; ICR mice, male, 25-30g, provided by the animal room of Xinzhong New Drug Research Center, China Pharmaceutical University.
[0069] 2 Methods and Results
[0070] 2.1 The impact on inferior vena cava thrombosis
[0071] 2.1.1 Effect of CSO on the formation of inferior vena cava thrombosis in mice
[0072] Fifty male Kunming mice, 25-30 g, fasted for 12 hours, were divided into 5 groups, 10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com