Expression of P27 protein and its use
A technology of p27 and protein, which is applied in the expression of p27 protein and its application field, which can solve the problems of cumbersome avian leukosis virus, high cost of plasma poisoning, complicated operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 P27 protein preparation process
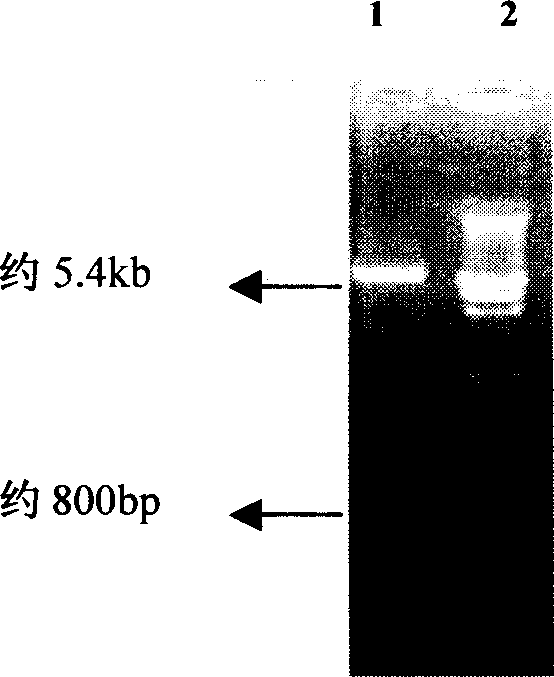
[0024] 1 Preparation of recombinant engineered bacteria
[0025] A recombinant engineering bacterium for expressing p27 protein was constructed, the expression vector used was pET28α, and the host bacterium was Escherichia coli BL21(DE3).
[0026] (1) Construction process of recombinant engineered bacteria
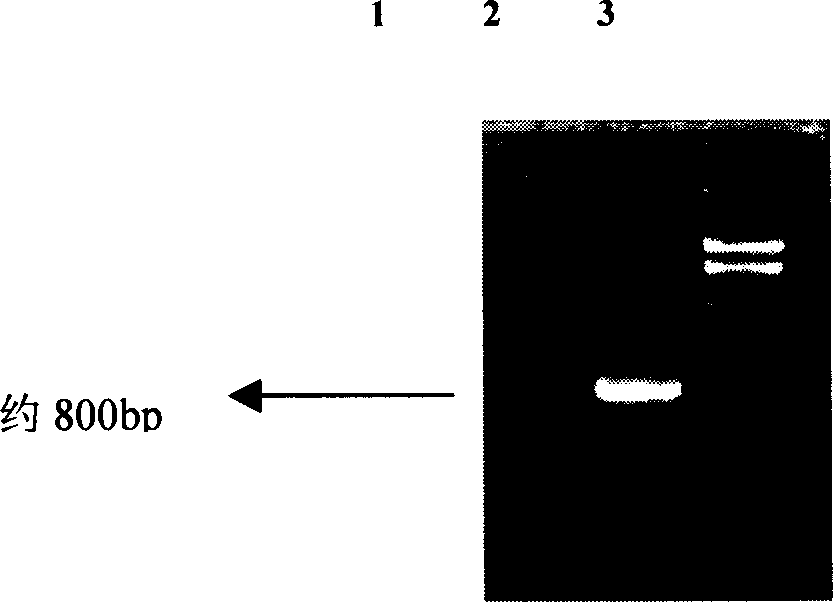
[0027] ① Preparation of the target fragment to be inserted
[0028] a) Double digestion of recombinant plasmid pGEM-Teasy / p27:
[0029] The recombinant plasmid pGEM-Teasy / p27 was double digested with BmaHI and SalI, and the reaction system was as follows:
[0030] The total reaction volume is 50 μl
[0031] BmaHI 2 μl
[0032] SalI 2μl
[0033] 10×T buffer 7.5μl
[0034] Substrate DNA 20μl
[0035] Sterilized water 18.5μl
[0036] The above components were mixed evenly, and after brief centrifugation, incubated at 37° C. for 6 hours.
[0037] b) recovery of enzyme digestion products:
[0038] Agarose gel electro...
Embodiment 2
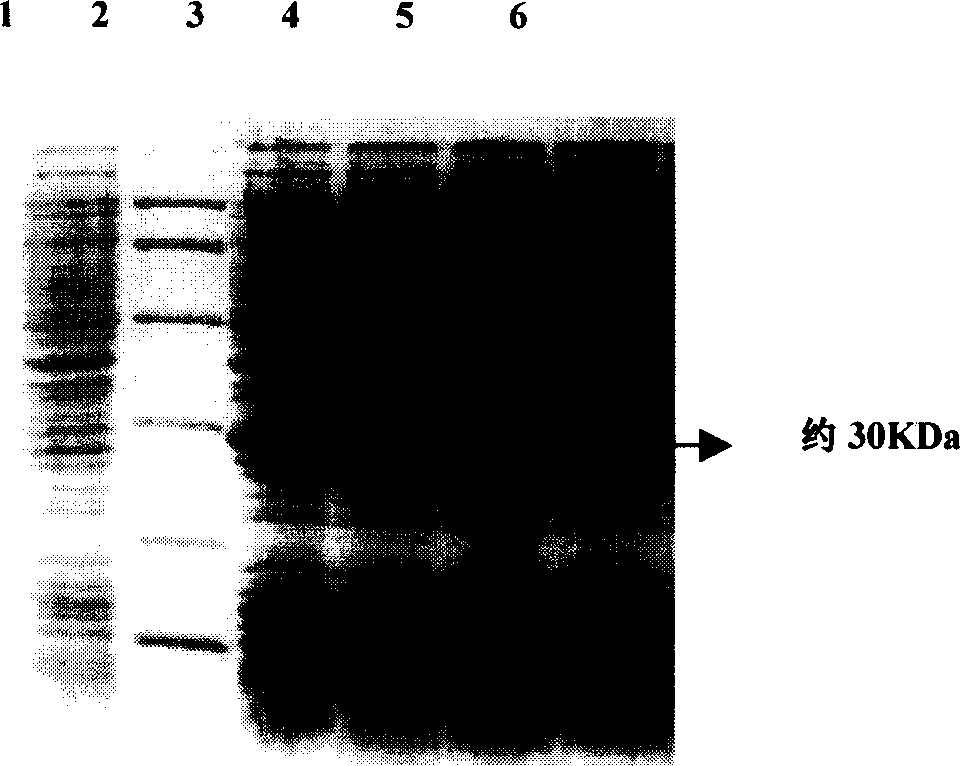
[0137] Example 2 Induced expression of p27 gene and purification of expression product
[0138] 1. Induced expression of p27 gene:
[0139] (1) Expression process
[0140] ①Select a well-growing positive single colony and inoculate it in 3ml LB (containing 30μg / ml Kana) medium, culture overnight at 37°C.
[0141] ② Take out 0.05ml and inoculate it into 5ml LB (containing 30μg / ml Kana) medium, and incubate at 30°C for 2-3 hours (OD600 to 0.4-0.6).
[0142] ③Add IPTG to a final concentration of 3mM, and induce expression at 30°C for 5 hours.
[0143] ④ Centrifuge at 5000rpm at 4°C for 10 minutes, discard the culture medium, collect the bacterial pellet, and store at -20°C.
[0144] (2) Reagents used for expression
[0145] ①Kanamycin (Kana) stock solution (30mg / ml): 300mg kanamycin was dissolved in 10ml sterilized water, sterilized by filtration, and dispensed at -20°C for use.
[0146] ② LB liquid culture medium Tryptone 10g, yeast extract 5g, NaCl 10g, dissolved in 950ml ...
Embodiment 3
[0242] Example 3 Preparation of rabbit anti-p27 antibody
[0243] (1) Immune acquisition of rabbit anti-P27 antibody
[0244] Rabbits were immunized with the prepared purified p27 protein by subcutaneous injection, intradermal injection and intramuscular injection.
[0245] 1. First immunization: Intradermal and intramuscular injection, the injection dose is 100μg / monkey. Take a certain amount of antigen solution, add an equal amount of Freund's complete adjuvant, emulsify completely, and inject 50 μg intradermally and intramuscularly.
[0246] 2. Second immunization: One week later, subcutaneous and intramuscular injections are used for the second immunization, and the injection dose is 200 μg / monkey. Take a certain amount of antigen solution, add the same amount of Freund's incomplete adjuvant, emulsify completely, and inject 100 μg subcutaneously and intramuscularly into each rabbit.
[0247] 3. Three immunizations: One week after the second immunization, the third immuniz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com