Compound of antitumor antibiotic of new carbon framework as well as preparation method and application
A compound and anti-tumor technology, applied in the field of medicine, can solve problems such as undiscovered patents or literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
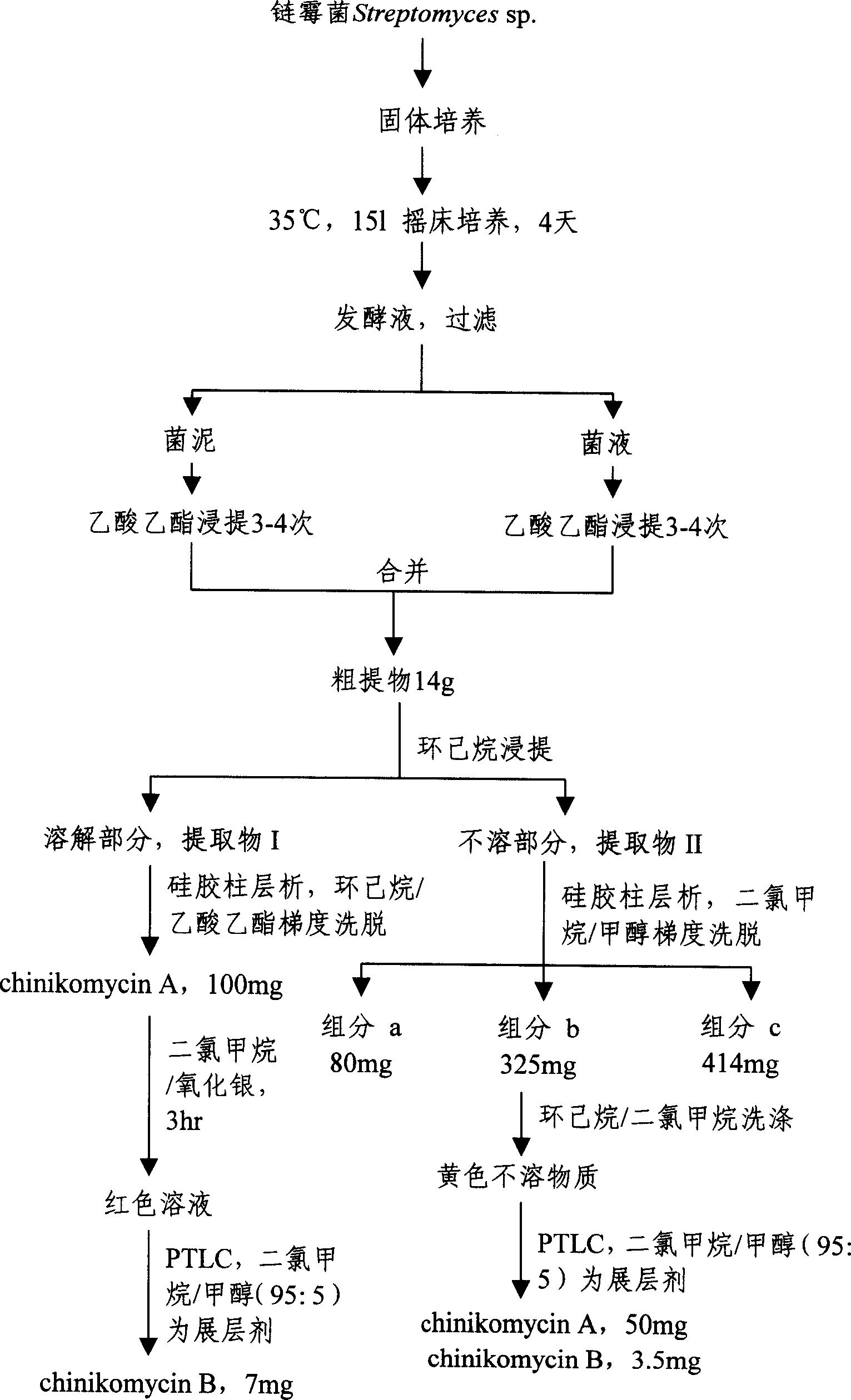
[0036] Preparation of new carbon-skeleton anti-tumor antibiotics chinikomycin A and B (for the preparation flow chart, see image 3 ):
[0037] (1) Sample collection: sea mud samples were collected from the coastal waters of Qingdao, and Gaoshi No. 1 medium was used in the laboratory (20 g of soluble starch, 1 g of potassium nitrate, 0.5 g of potassium dihydrogen phosphate, 0.5 g of magnesium sulfate, and 0.5 g of sodium chloride) g, ferrous sulfate 0.01g, potassium dichromate 0.1g, agar powder 18g, natural seawater 500ml, tap water 500ml, adjust pH to be 7.2) carry out gradient dilution streak culture, purify and obtain a strain of marine Streptomyces sp.;
[0038] (2) Solid culture: the marine Streptomyces sp. was inoculated on M 2+ Solid medium (barley extract 10g, yeast powder 4g, glucose 4g, natural seawater 500ml, tap water 500ml, agar powder 14g, adjust pH to 7.4), culture at 28°C for 3 days until white spores grow;
[0039] (3) shaker culture: from the M 2+ Pick whi...
Embodiment 2
[0100] Oxidation from chinikomycin A to chinikomycin B:
[0101] Get the product chinikomycin A of 20mg, join in the reaction bottle, dissolve with 50ml dichloromethane (this embodiment just dissolves), slowly add about 1g of silver oxide (it is excessive with respect to the chinikomycin A in the solution), stir , reacted for about 3 hours, the color of the solution changed from yellow to red completely, and was spun to dryness, dissolved with 6ml of dichloromethane (this embodiment was fully dissolved), discarded the precipitated insoluble matter, and the dissolved part was subjected to preparative thin-layer chromatography ( PTLC), with 95% dichloromethane / 5% methanol as developing solvent, reclaim R f = 7 mg of the red compound at 0.58, the chemical structure of the compound was determined as chinikomycin B through proton nuclear magnetic resonance, carbon nuclear magnetic resonance, mass spectrometry, infrared and ultraviolet spectrometry.
Embodiment 3
[0103] Reduction of chinikomycin B to chinikomycin A:
[0104] In the test tube, add 0.5mg of chinikomycin B, dissolve it with 2ml of dichloromethane, then slowly add 1ml of 0.1N sodium dithionite solution, shake until the color of the solution changes from red to yellow, and analyze it by thin layer chromatography (TLC). R f The yellow compound at =0.34 is chinikomycinA.
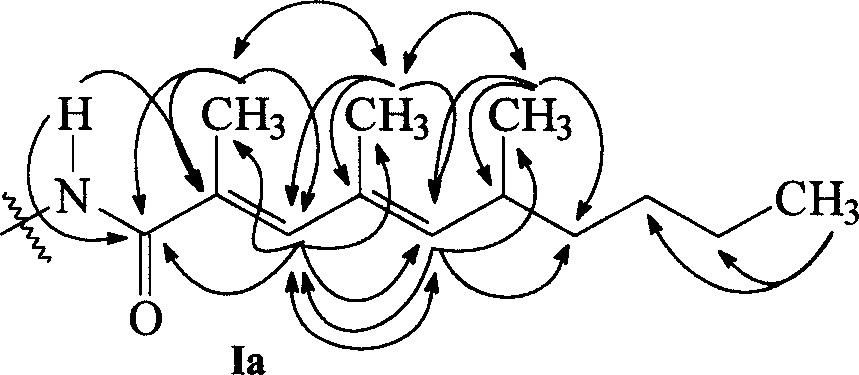
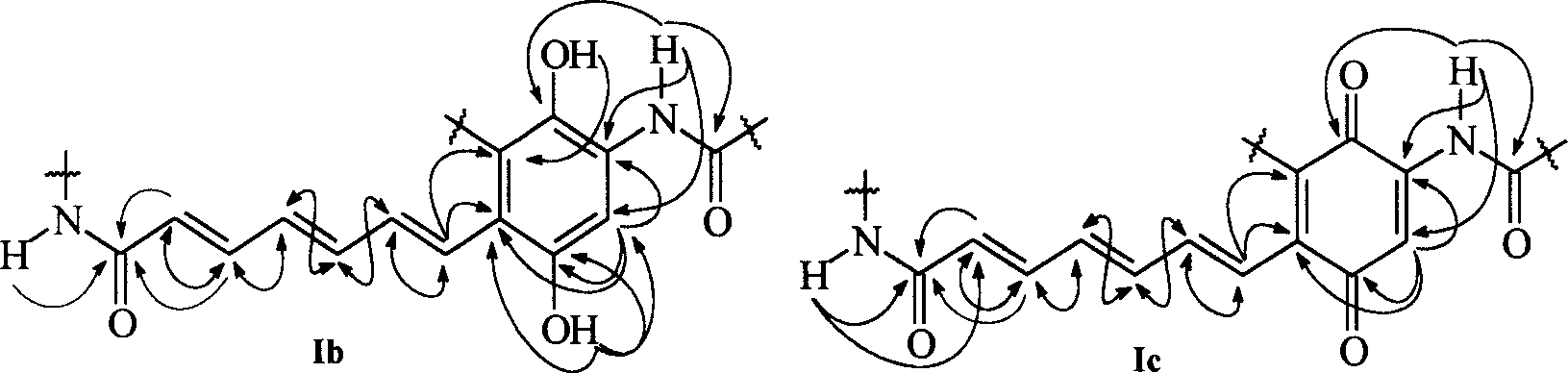
[0105] Two-dimensional NMR analysis of compounds chinikomycin A and chinikomycin B further verified the structures of compounds chinikomycin A and chinikomycin B. The HMBC (→) and NOESY () effects of the hydrocarbon amide side chains can be found in figure 1 , HMBC(→) and H, H COZY() effects of the carbon skeleton see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com