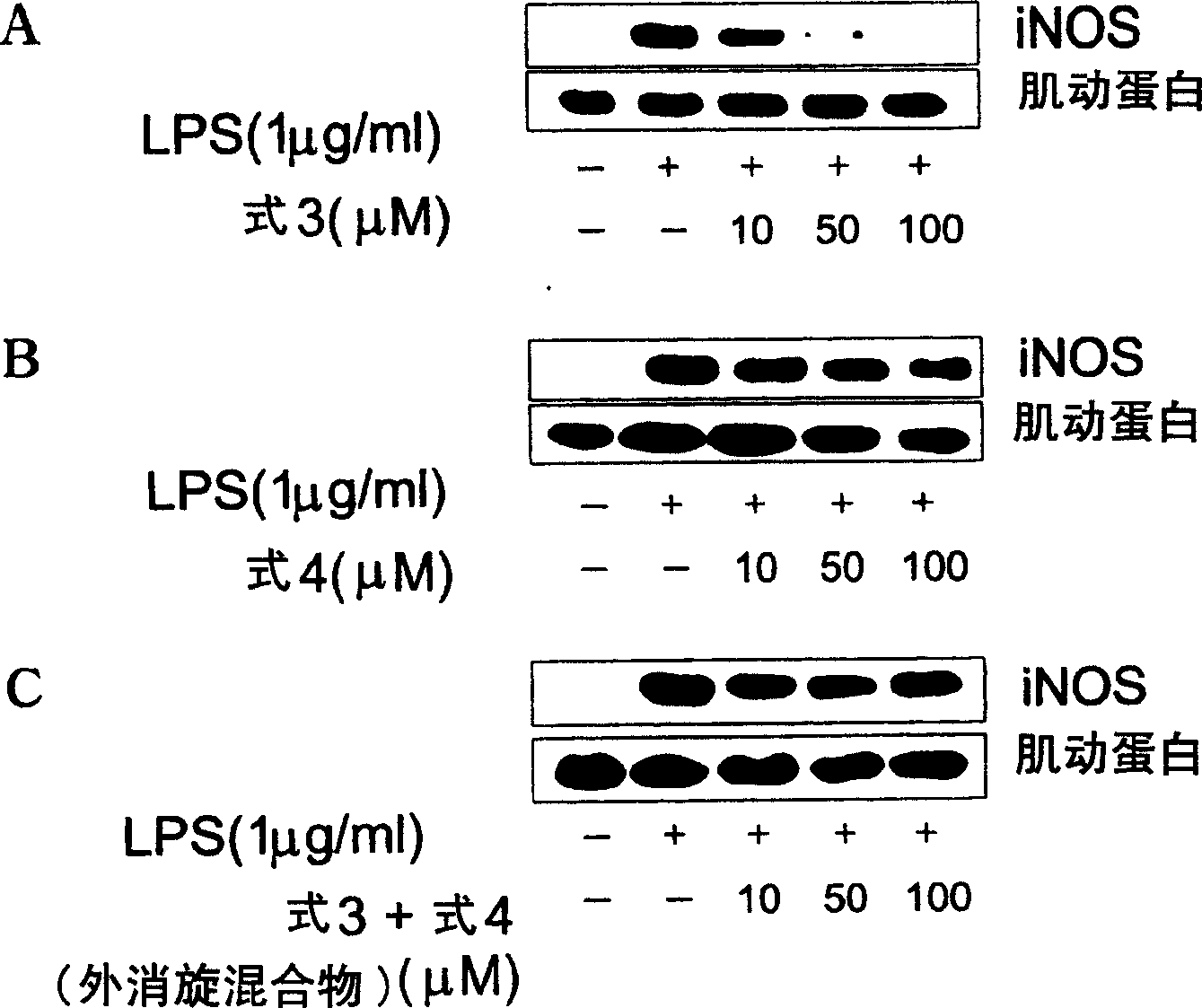
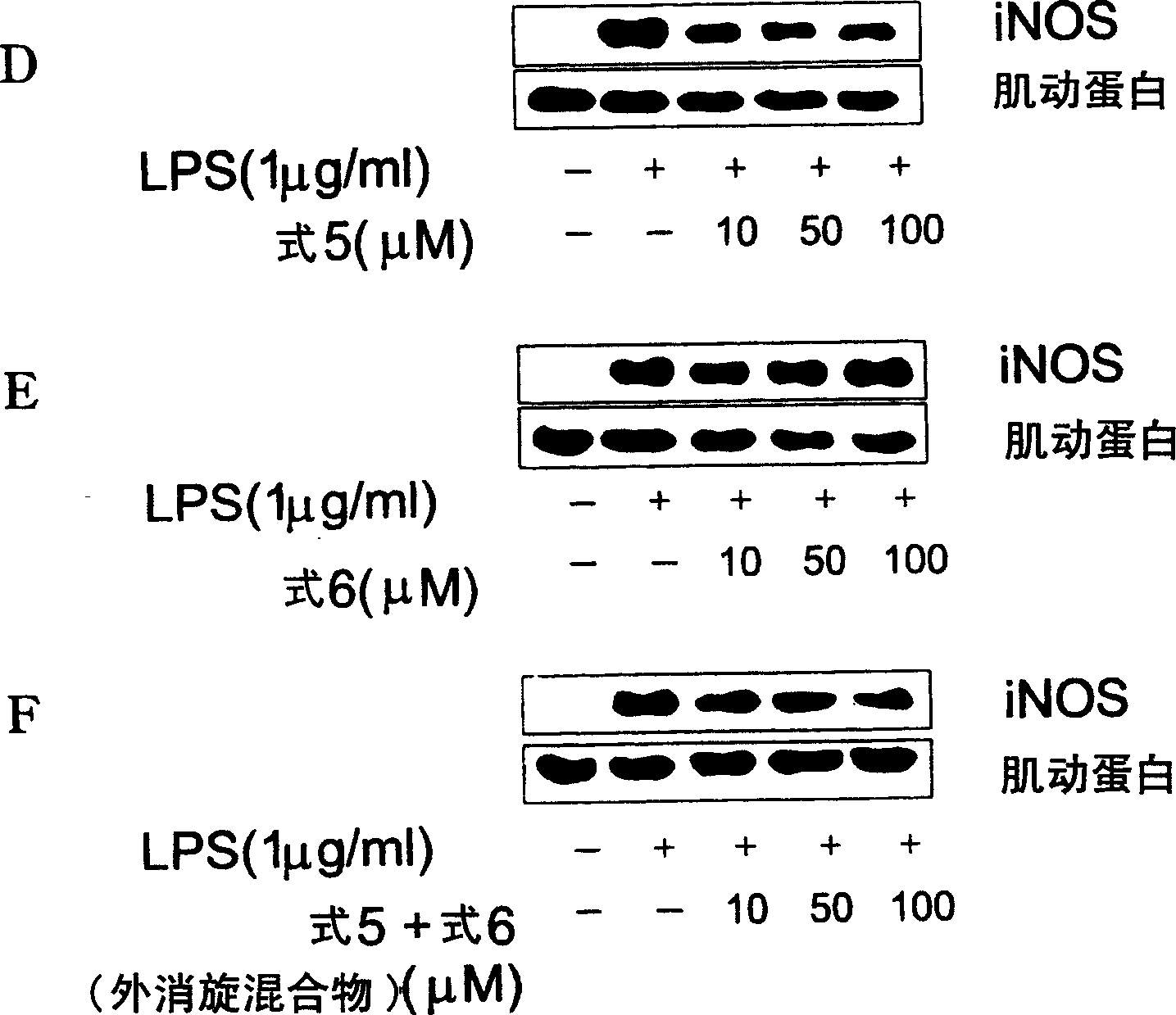
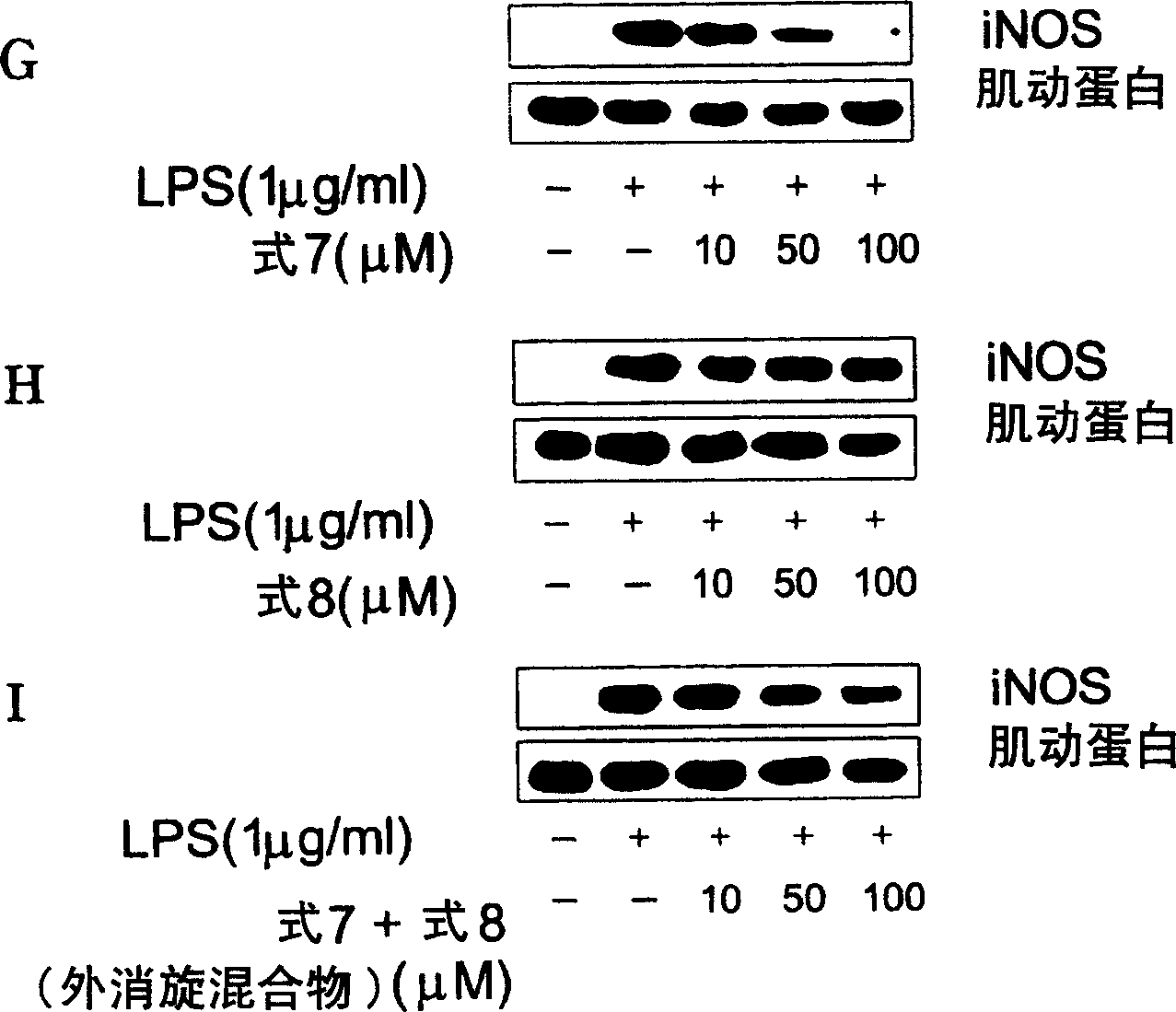
Novel enantiomer of tetra hydrogen isoquinoline derivative and officinal salt, preparation and pharmaceutical composition thereof
A technology of tetrahydroisoquinoline and derivatives, applied in the field of new enantiomers, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0201] Two-μ-chloro-bis[(η 6 - p-cymene) ruthenium (II) chloride]
[0202] [Reaction Scheme 1]
[0203]
[0204] In a flask, the RuCl 3 h 2 O (514 mg, 1.97 mmol) was dissolved in ethanol (25 ml), to which α-phellandrene (3.51 mL, 21.6 mmol) was added dropwise. Then, the flask equipped with a reflux condenser was filled with nitrogen. Using a thermostat, the temperature of the reaction solution was adjusted to 79° C., followed by reflux for 4 hours. The reaction mixture was cooled to room temperature. After this time, the precipitated solid was filtered through a Buchner funnel. The brown solid thus obtained was washed once with methanol (40 ml), and the solvent was removed under reduced pressure. The brown solid (340 mg) was recrystallized from methanol (3 mL) to provide the desired compound, di-μ-chloro-bis[(η 6 - p-cymene) ruthenium (II) chloride] (211 mg, 35%) as a brown solid.
[0205] 1 H-NMR (300MHz, DMSO-d 6 ): δ5.77(q, 4H), 2.8(m, 1H), 2.1(s, 3H), 1.2(d, ...
preparation Embodiment 2
[0206] Synthesis of RuCl[TsDPEN] (p-cymene) catalyst
[0207] [Reaction Route 2]
[0208]
[0209] In a flask, di-μ-chloro-bis[(η 6 - p-cymene) ruthenium (II) chloride] (211 mg, 345 μmol) was dissolved in 2-propanol (10 mL), to which was added triethylamine (TEA) (0.192 mL, 1.38 mmol), then dropwise Add (1S,2S)-(p-toluenesulfonyl)-1,2-diphenylethylenediamine (253 mg, 689 μmol). Then, the flask equipped with a reflux condenser was filled with nitrogen. Using a thermostat, the temperature of the reaction solution was adjusted to 80° C., followed by reflux for 1.5 hours. Reaction completion was checked by thin layer chromatography. The reaction mixture was cooled to room temperature and concentrated in vacuo to afford a very viscous liquid residue. The residue was dissolved in methanol (1 ml) with gentle heating, and allowed to stand for 1 day and night. A dark red solid precipitated and only the dark brown supernatant was discarded. The residue of the dark red solid p...
Embodiment 1
[0212] Synthesis of (R)-6,7-dihydroxy-1-(p-hydroxybenzyl)-1,2,3,4-tetrahydroisoquinoline hydrobromide
[0213] (Step 1): Synthesis of N-(3,4-dimethoxyphenethyl)(p-methoxyphenyl)acetamide
[0214] [Reaction Route 3]
[0215]
[0216] To p-methoxyphenylacetic acid (50.4 g, 0.303 mol) in a 50 ml round bottom flask was added 3,4-dimethoxyphenethylamine (51.2 mL, 0.303 mol) dropwise. Then, the flask equipped with a reflux condenser was filled with nitrogen. Using a thermostat, the temperature of the reaction solution was adjusted to 198-200° C. and heated for 4 hours. Reaction completion was checked by thin layer chromatography. The reaction mixture was cooled to room temperature, and chloroform (500 mL) was added thereto to dissolve the synthesized precipitate. Continuously with 1N HCl, 1N NaHCO 3 The chloroform solution was washed with saturated brine, dried over anhydrous magnesium sulfate, and filtered through a glass filter. The filtrate was concentrated in vacuo to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com