Method for synthesizing series single-dispersed ferrite nanometer magnetic beads
A technology of nano-magnetic beads and ferrite, which is applied in the direction of nanotechnology, nanotechnology, nanostructure manufacturing, etc., can solve the problems of insufficient dispersion and uneven product size distribution, and achieve large performance control space and product shape Uniform, broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
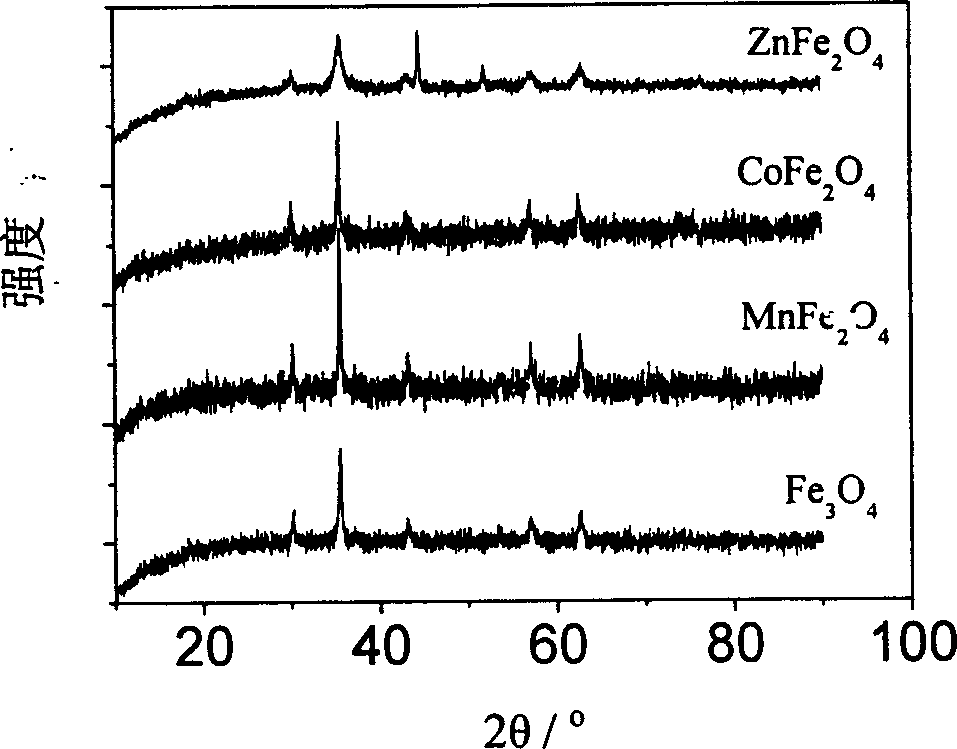
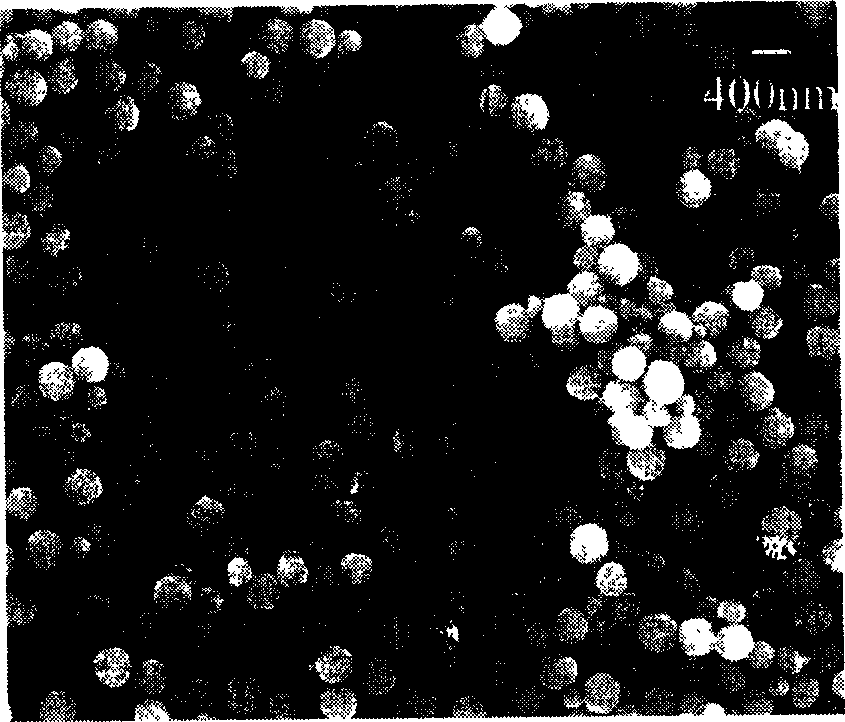
[0036] Take 16mmol of FeCl 3 , added to a 50 ml hydrothermal kettle, 40 ml of ethylene glycol solution was added to the kettle, after dissolving, heated at 200°C for 72 hours, the obtained black precipitate was washed with deionized water, dried at 40-80°C, and prepared Ferric oxide nano-magnetic beads with a particle diameter of 100-1000 nanometers are obtained.
[0037] Reduce FeCl 3 The usage amount is up to 2mmol, and a similar product is prepared through the same process;
[0038] Ferric sulfate, ferric nitrate, and ferric acetate are used to replace ferric chloride, and similar products are obtained through the same process.
Embodiment 2
[0040] Take 8mmol of manganese chloride and 16mmol of FeCl 3 Added to 50 ml of water heating kettle, at this time Mn 2+ with Fe 3+ The molar ratio is 1:2. Add 40 ml of ethylene glycol solution to the kettle, stir and heat at 250°C for 18 hours, wash the obtained precipitate with deionized water, and dry it at 40-80°C to obtain MnFe 2 o 4 nano magnetic beads.
[0041] Control the molar ratio of divalent metal ions to iron ions to be 1:2, the same process is applicable to Ni2+ , Cu 2+ , Zn 2+ , Cd 2+ , Pb 2+ , Sn 2+ , Ca 2+ ,Sr 2+ , Ba 2+ , Cd 2+ , Mg 2+ ,Co 2+ The reaction of soluble salts of divalent metal ions and soluble ferric ion salts, finally forming NiFe 2 o 4 , CuFe 2 o 4 , ZnFe 2 o 4 , CdFe 2 o 4 , PbFe 2 o 4 , SnFe 2 o 4 , CaFe 2 o 4 , SrFe 2 o 4 , BaFe 2 o 4 , CdFe 2 o 4 , MgFe 2 o 4 , CoFe 2 o 4 and other ferrite nano-magnetic beads.
Embodiment 3
[0043] Weigh 0.08mmolMnCl 2 and 16mmol of FeCl 3 , added to a 50 ml water heating kettle, at this time Mn 2+ with Fe 3+ The molar ratio is 0.01:2, 40 ml of ethylene glycol solution is added to the kettle, heated at 300°C for 8 hours, the obtained precipitate is washed with deionized water, and dried at 40-80°C to obtain Mn x Fe 3-x o 4 Manganese ferrite compound nano magnetic beads.
[0044] control Mn 2+ The molar ratio to iron ions is x:2, where 0.01<x<1, and manganese ferrite nano magnetic balls with other ratios are prepared.
[0045] Control the molar ratio of divalent metal ions to iron ions to be x: 2, where 0.12+ , Cu 2+ , Zn 2+ , Cd 2+ , Pb 2+ , Sn 2+ , Ca 2+ ,Sr 2+ , Ba 2+ , Cd 2+ , Mg 2+ ,Co 2+ The reaction of soluble salts of divalent metal ions and soluble ferric ion salts, and finally the formation of Ni x Fe 3-x o 4 , Cu x Fe 3-x o 4 , Zn x Fe 3-x o 4 , Cd x Fe 3-x o 4 , Pb x Fe 3-x o 4 , Sn x Fe 3-x o 4 , Ca x Fe 3-x o 4 ,S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com