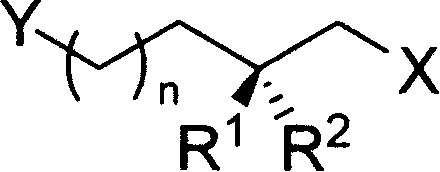
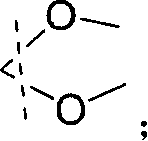
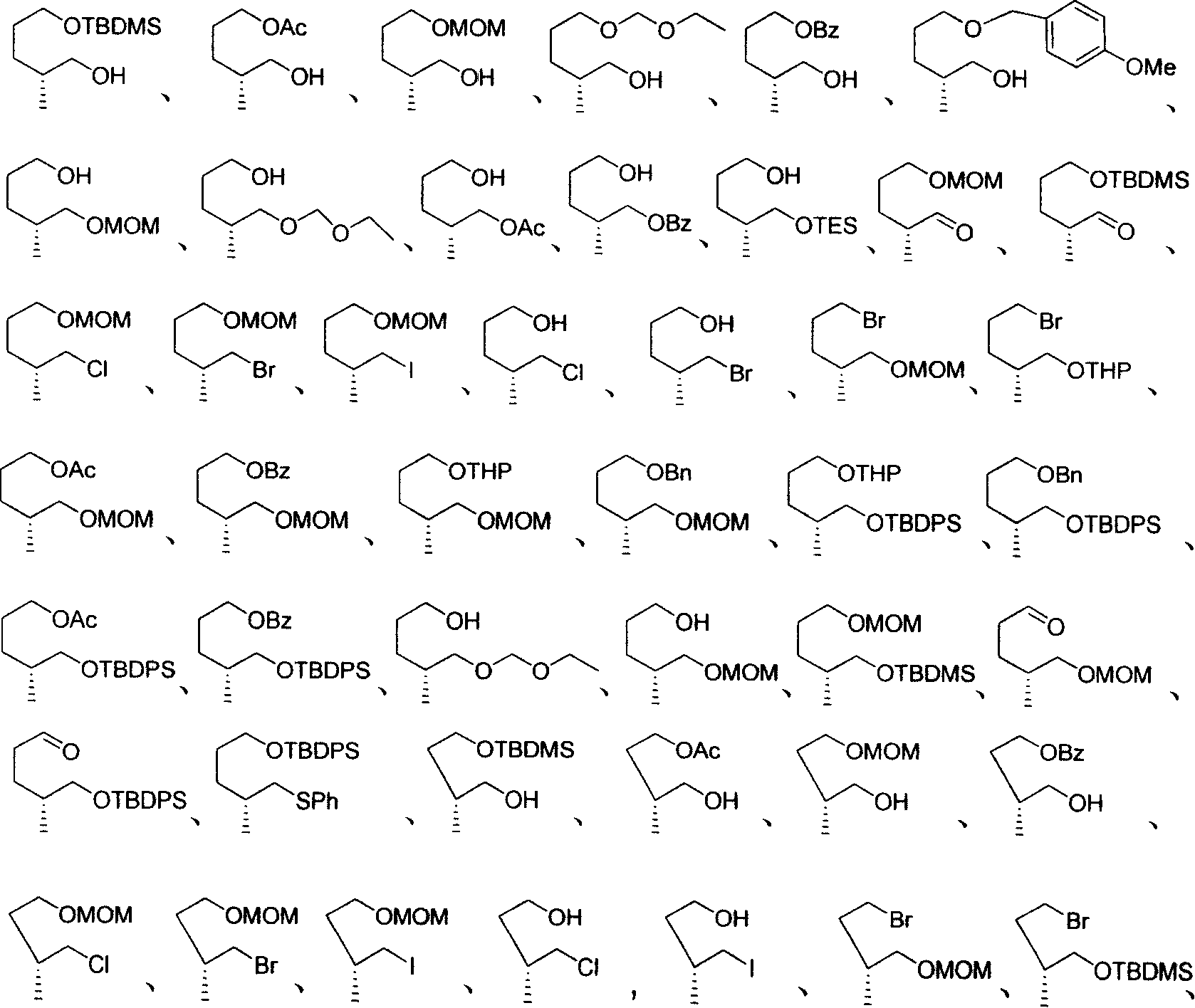
2-ramification of methyl alkyl diol in optical purity
A technology of methyl alkyl diols and derivatives, which is applied in the directions of organic chemistry, chemical instruments and methods, and compounds of elements of Group 4/14 of the periodic table, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107]
[0108] R 1 = H, R 2 = Me or R 1 = Me,R 2 =H, n=1 or 2, PG is a hydroxyl protecting group
[0109] 2R-5-tert-butyldimethylsilyloxy-2-methyl-1-pentanol:
[0110] At room temperature, 11.4 grams of 4R-4-methyl-valerolactone as a raw material was dissolved in 100 ml of anhydrous tetrahydrofuran, and 8 grams of LiAlH 4 200ml of anhydrous tetrahydrofuran suspension, after the reaction is complete. The reaction was extracted with ethyl acetate, then water was added dropwise until the solid was white, the white solid was removed by filtration, the residue was fully washed with tetrahydrofuran, the solvent was spinned off under reduced pressure, and then distilled under reduced pressure to obtain 2R-2-methyl-1,5 - 10.8 grams of pentanediol (80°C / 0.1mmHg, 92%)
[0111] 1.2 g of 2R-2-methyl-1,5-pentanediol was dissolved in 10 ml of N, N-dimethylformamide, 1.5 equivalents of imidazole and 1.2 equivalents of TBDMSCl were added, stirred at room temperature for 10 ...
Embodiment 2
[0134]
[0135] R 1 = H, R 2 = Me or R 1 = Me,R 2 =H, n=0 or 1, PG is a hydroxyl protecting group
[0136] 4R-5-methoxymethoxy-4-methyl-1-pentanol:
[0137] At room temperature, 11.4 g of the raw material 4R-4-methyl-valerolactone was dissolved in 100 ml of anhydrous methanol, 0.8 mL of concentrated sulfuric acid was added, and refluxed for 2 hours, after the reaction was completed. Extract with ether, saturated NaHCO 3 Solution washing, water washing, MgSO 4 After drying, the solvent was spin-dried under reduced pressure at low temperature to obtain 14.4 g (99%) of 4R-4-methyl-5-hydroxy-valeric acid methyl ester. 1.4 g of 4R-4-methyl-5-hydroxy-pentanoic acid methyl ester was dissolved in 10 ml of dichloromethane, and 1.5 equivalents of DBU (1,8-diazabicyclo[5,4,0]undec-7-ene) was added , 1.2 equivalents of chloromethoxymethane, stirred at room temperature for 5 hours, the product was extracted with dichloromethane, washed twice with water, MgSO 4 dry. Spin in...
Embodiment 3
[0156]
[0157] R 1 = H, R 2 = Me or R 1 = Me,R 2 =H, n=1 or 2, Y=halogen
[0158] 4R-5-bromo-1-methoxymethoxy-4-methylpentane:
[0159] 19 g of 4R-5-methoxymethyl-4-methyl-1-pentanol was dissolved in 100 mL of dichloromethane, and 1.5 unit volume of PPh was added 3 and 1.5 single quantities of CBr 4 , stirred at room temperature for 4 hours, the product was washed with saturated sodium bicarbonate solution, washed twice with water, MgSO 4 Dry and spin off the solvent. Distillation under reduced pressure at 40°C / 0.2 mmHg yielded 21.2 g of a colorless oil. Yield 95%.
[0160] C8H17BrO2: (225.12)
[0161] Elemental analysis: C%=42.13; H%=7.65; Mass spectrum: (M + , 224)
[0162] 1 H-NMR (CDCl 3 ): δ4.64(s, 2H), 3.4(m, 4H), 3.36(s, 3H), 1.59(m, 5H), 0.95(d, J=6Hz, 3H)
[0163] Made in a similar way:
[0164] 3R-4-bromo-1-methoxymethoxy-3-methylbutane: yield 94%, mass spectrum: (M + ,210);
[0165] 3R-4-bromo-1-ethoxymethoxy-3-methylbutane: yield 92%, m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com