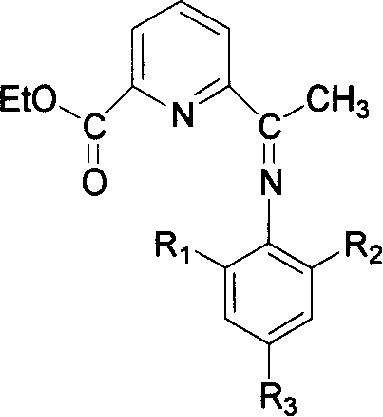
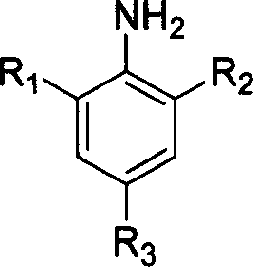
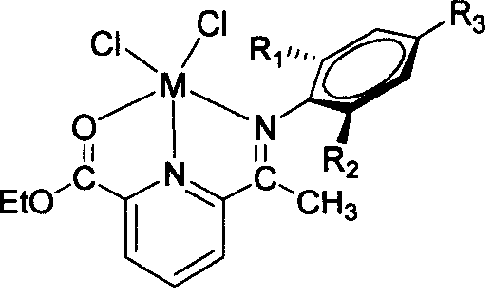
Compound of monoimine pyridine, preparation method and catalyst containing the compound and application
A technology of monoiminopyridine and compound, which is applied in the field of monoiminopyridine compound to achieve the effects of improved yield, high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Synthesis of monoimine pyridine compound precursor 2-acetyl-6-ethoxypyridine:
[0035] Using 2,6-lutidine as raw material, 2,6-dicarboxylic acid pyridine was prepared by oxidation of potassium permanganate (Singer, A.W.; McElvain, S.M.J.Am.Chem.Soc.1935, 57, 1135. Beilstein Handbook of Organic Chemistry, fifth Supplementary Series, Springer-Verlag, Berlin, 1990, Vol.22, part 4, 128.), and then prepare 2,6-dicarboxypyridine by esterification. The preparation procedure was simplified from the literature method and gave a higher yield (83%) than the literature value (60%) (Barnes, R.A.; Fales, H.M.J. Am. Chem. Soc. 1953, 75, 3830.). Then 2,6-dicarboxypyridine was dissolved in freshly distilled EtOAc, treated with dry sodium alkoxide powder, and then refluxed with excess concentrated hydrochloric acid to finally obtain the product 2-acetyl-6-carboxypyridine .
[0036] Sodium flakes (2.10 g, 91 mmol) were added into 60 mL of absolute ethanol. After the sodium flakes wer...
Embodiment 2
[0044] 1. Synthesis of monoimine pyridine compound 2-carboethoxy-6-{1-[(2,4,6-trimethylphenyl)imine]ethyl}pyridine:
[0045] Add 2-acetyl-6-carboethoxypyridine (0.772g, 4.0mmol) and excess 2,4,6-trimethylaniline (1.08g, 8.0mmol) prepared in Example 1 into a 25mL conical flask middle. The Erlenmeyer flask was put into a microwave oven (Media), and reacted for 20 minutes under high fire (800W) conditions. The obtained crude product was purified by chromatography (silica gel column 60×2.5 cm, eluent: petroleum ether:ethyl acetate=2:1). A light yellow solid was obtained with a yield of 74.1%. mp 75.0-76.0℃. 1 H NMR (400MHz, CDCl 3 ): δ8.56(d, 1H, Py-Hm), 8.19(d, 1H, Py-Hm), 7.94(t, 1H, Py-Hp), 6.90(s, 2H, Ar-Hm), 4.88( m, 2H, CH 2 ), 2.26(d, 3H, N=C(Me)), 2.06(s, 6H, Ar-Me), 1.47(t, 3H, CH 2 (CH 3 )).IR(KBr):ν C=O 1715cm -1 , ν C=N 1643cm -1 , ν C-O-C 1137cm -1 .Elemental analysis C 19 h 22 N 2 o 2 Theoretical value: C, 73.52; H, 7.14; N, 9.03. Found value: C, 73...
Embodiment 3
[0051] 1. Synthesis of monoimine pyridine compound 2-carboethoxy-6-{1-[(2,6-diethylphenyl)imine]ethyl}pyridine:
[0052] Add 2-acetyl-6-carboethoxypyridine (0.670 g, 3.5 mmol) and excess 2,6-diethylaniline (1.07 g, 7.2 mmol) prepared in Example 1 into a 25 mL Erlenmeyer flask. The Erlenmeyer flask was put into a microwave oven (Media), and reacted for 35 minutes under high fire (800W) conditions. The obtained crude product was separated by chromatography (silica gel column 30×1.5cm, eluent: n-hexane:dichloromethane=1:2, silica gel column 40×1.5cm, eluent: ether:petroleum ether=2:3), The product was purified to obtain a light yellow solid with a yield of 17.6%. mp84.0-86.0℃. 1 H NMR (400MHz, CDCl 3 ): δ8.48(d, 1H, Py-Hm), 8.11(d, 1H, Py-Hm), 7.87(t, 1H, Py-Hp), 7.30(d, 2H, Ar-Hm), 6.99( t, 1H, Ar-Hp), 4.43 (m, 2H, COOCH 2 ), 2.28(m, 4H, (CH 2 )CH 3 ), 2.20(s, 3H, N=C(Me)), 1.39(t, 3H, COOCH 2 (CH 3 )), 1.05(t, 6H, CH 2 (CH 3 )).IR(KBr):ν C=O 1742.3cm -1 , ν C=N 16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com