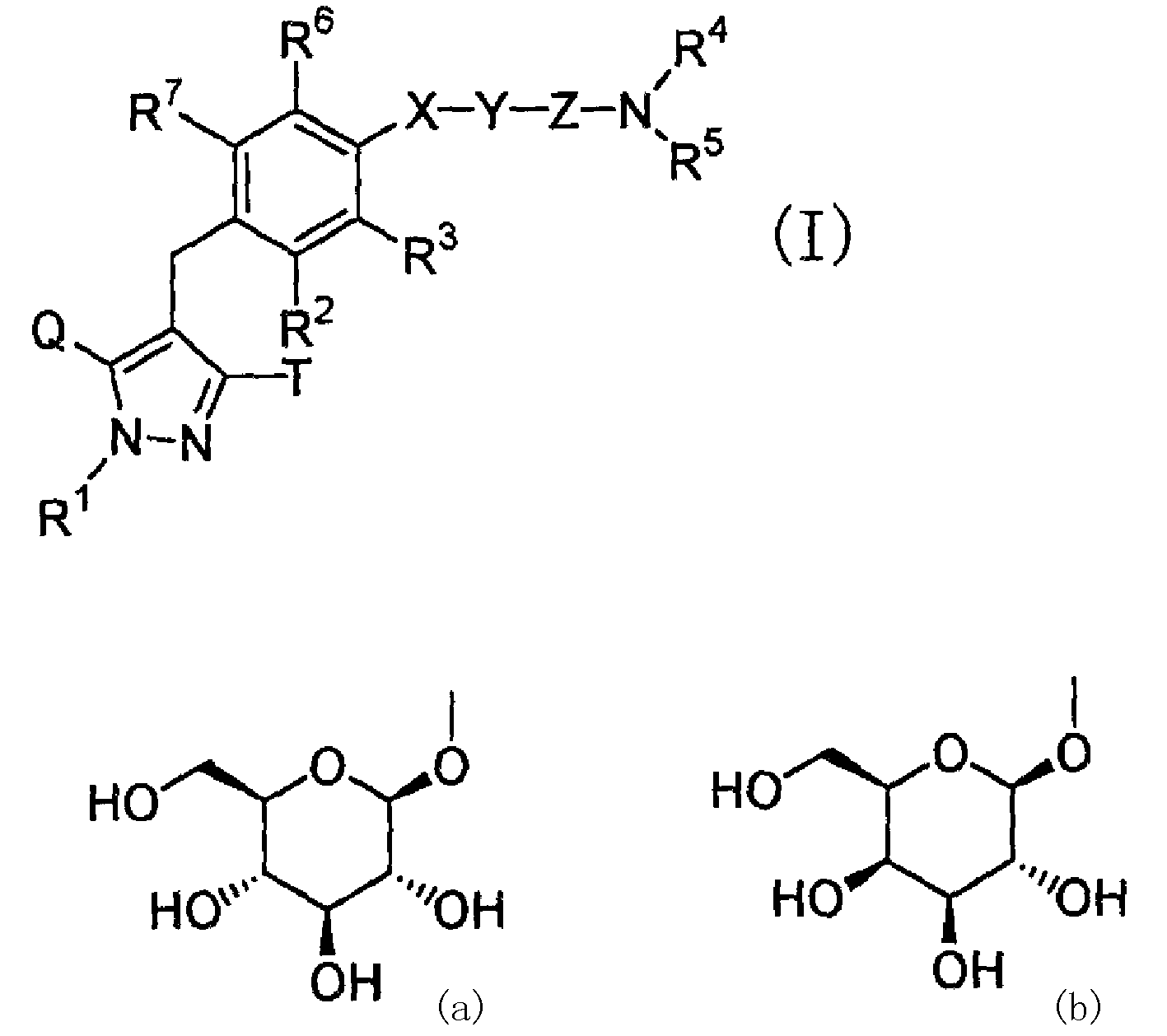
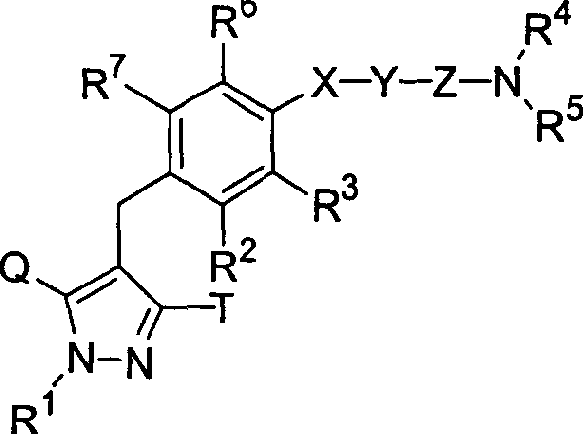
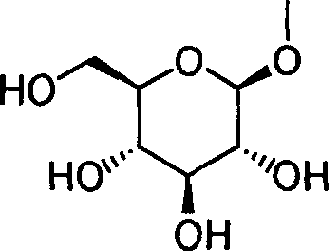
Pyrazole derivative, medicinal composition containing the same, medicinal use thereof, and intermediate for production thereof
A technology of pyrazole derivatives and alkyl, applied in the field of pyrazole derivatives, can solve the problems of insufficient absorption of glucose and galactose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0303] 4-({4-[3-(carbamoylmethylcarbamoyl)propyl]phenyl}methyl)-3-(β-D-glucopyranose Oxy)-5-isopropyl-1H-pyrazole
[0304] To 3-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy)-4-({4-[(1E)-3-carboxyprop-1-ene base]phenyl}methyl)-5-isopropyl-1H-pyrazole (0.34g) in N,N-dimethylformamide (1mL) solution, add glycinamide hydrochloride (0.12g ), 1-hydroxybenzotriazole (0.09g), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (0.15g) and triethylamine (0.27g) , and the mixture was stirred overnight at room temperature. Insoluble material was removed by filtration. To the filtrate, 5 mol / L aqueous sodium hydroxide solution (0.5 mL) was added, and the mixture was stirred at room temperature for 1 hr. The insoluble matter was removed by filtration, and then the filtrate was purified by preparative reverse column chromatography (Shiseido CAPCELL PAK UG120 ODS, 5 μm, 120 Å, 20×50 mm, flow rate 30 mL / min linear gradient, water / acetonitrile=90 / 10-10 / 90), giving 4-({4-[(1E)...
Embodiment 2
[0308] 4-{[4-(3-carbamoylpropyl)phenyl]methyl}-3-(β-D-glucopyranosyloxy)-5-isopropyl- 1H-pyrazole
[0309] The title compound was prepared analogously to Example 1, using ammonium chloride instead of glycinamide hydrochloride.
[0310] 1 H-NMR (CD 3 OD)δppm:
[0311] 1.05-1.2(6H, m), 1.8-1.95(2H, m), 2.19(2H, t, J=7.6Hz), 2.58(2H, t, J=7.5Hz), 2.85-2.95(1H, m) , 3.3-3.45(4H, m), 3.6-3.8(3H, m), 3.8-3.9(1H, m), 5.0-5.1(1H, m), 7.0-7.15(4H, m)
Embodiment 3
[0313] 4-({4-[3-(2-carbamoylethylcarbamoyl)propyl]phenyl}methyl)-3-(β-D-glucopyranose (Sugaroxy)-5-isopropyl-1H-pyrazole
[0314] The title compound was prepared analogously to Example 1, substituting 3-aminopropanamide for glycinamide hydrochloride.
[0315] 1 H-NMR (CD 3 OD)δppm:
[0316] 1.05-1.2(6H, m), 1.8-1.95(2H, m), 2.15(2H, t, J=7.3Hz), 2.4(2H, t, J=6.7Hz), 2.56(2H, t, J= 7.5Hz), 2.85-2.95(1H, m), 3.25-3.45(6H, m), 3.6-3.9(4H, m), 5.0-5.1(1H, m), 7.0-7.15(4H, m)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com