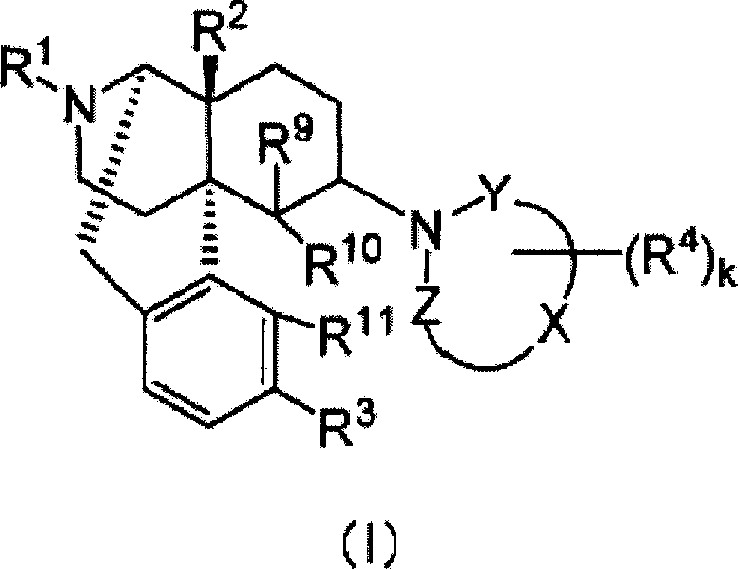
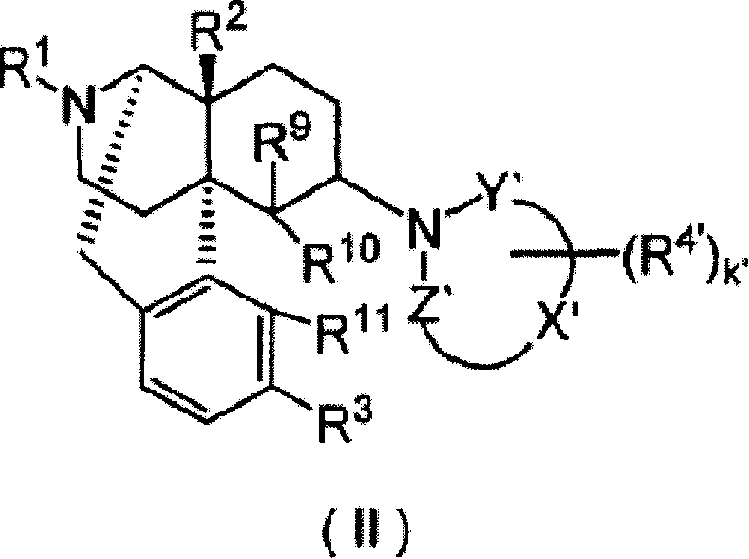
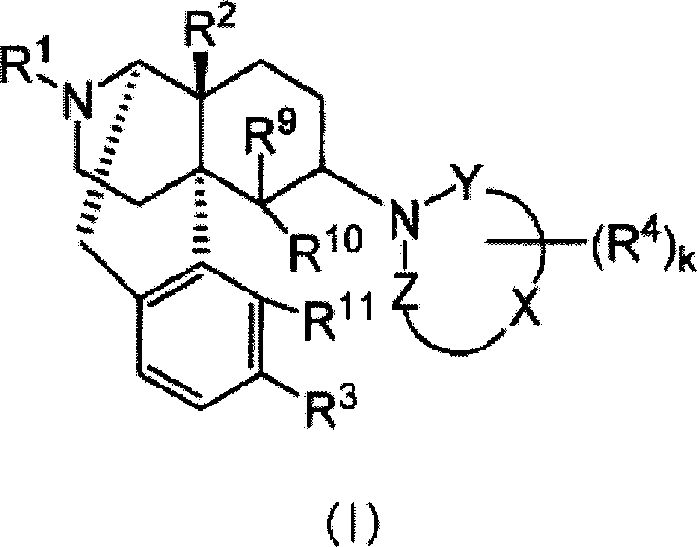
Remedies or preventives for urinary frequency or urinary incontinence and morphinan derivatives having nitrogen-containing heterocyclic group
A technology of substituents and urinary incontinence, applied in organic chemistry, drug combination, urinary system diseases, etc., can solve problems such as inability to use therapeutic or preventive agents, no disclosure, drug dependence and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0167] The present invention will be described in more detail below by way of examples.
[0168] Reference example 1-1
[0169] Synthesis of 6β-dibenzylamino-17-cyclopropylmethyl-4,5α-epoxy-morphinan-3,14-diol
[0170]
[0171] 249.8 g (0.731 mol) of naltrexone (Naltrexone) was dissolved in a mixed solvent of 1,700 ml of THF and 1,700 ml of toluene, and 432.7 g (2.193 mol, 3.0 equivalents) of dibenzylamine was added. The pressure in the reaction vessel was reduced while stirring, and replacement with argon was performed. Then, 357.7 g (2.929 mol, 4.0 equivalents) of benzoic acid was weighed and put into a beaker, slowly added to the solution, and a white solid was precipitated. Heating of the reaction apparatus was started with an oil bath, and as the internal temperature rose, the precipitated crystals were dissolved to form a homogeneous solution. Reflux began at an internal temperature of 81.5°C, and the reaction began. The reaction is carried out at an internal temp...
reference example 1-2
[0176] Synthesis of 6β-naltrexamine (naltrexamine)
[0177]
[0178] Weigh 325.0 g (0.622 mol) of 6β-dibenzylamino-17-cyclopropylmethyl-4,5α-epoxy-morphinane-3,14-diol obtained in Reference Example 1-1, 10% 65.0 g (20% wt) of Pd / C (50% humidity) was put into a 5L reaction vessel and installed on the reaction device. Then, methanol 2561.3g (3.25L, 10mL / g raw material) was added and stirring was started, and argon replacement was performed 3 times. Weigh 91.0 g (1.740 mol, 2.8 equivalents) of formic acid (88% solution) into a beaker, and add dropwise at an internal temperature of 22.1 to 25.8° C. over 5 minutes using a dropping funnel. At this time, it was confirmed that the internal temperature rose and gas was generated. Heating was started after completion|finish of dripping, and reaction started when internal temperature reached 51.1 degreeC, and it analyzed by HPLC 2 hours after reaction start. The reaction was terminated in 2 hours. To proceed the reaction, stirring ...
Embodiment 1-1
[0181] 4,5α-epoxy-6β-tetrahydroquinolino-3-methoxy-17-methyl-morphinane (compound 201 )Synthesis
[0182]
[0183] 201
[0184] Dissolve 304g (1.02mmol) of dihydrocodone and 0.12ml (1.65mmol) of methanesulfonic acid in a mixed solvent of 20ml xylene and 10ml dimethylformamide, add 1,2,3,4-tetrahydro After 0.2ml (1.59mmol) of quinoline was removed with a 175°C oil bath, the water was azeotropically removed and heated to reflux for 12 hours. After the reaction solution was left to cool to room temperature, 50 ml of saturated aqueous sodium bicarbonate solution and 3 ml of ammonia water were added to the reaction solution, and extracted with chloroform (50 ml×3). The organic layers were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, and concentrated to obtain 309 mg of a crude product. The obtained crude product was dissolved in 20 ml of methanol, 1.014 g (16.1 mmol) of sodium cyanoborohydride was added, and then 0.17 ml (2.62 mmol) of metha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com