Injectable long-acting microsphere suspension contg.
A technology of huperzine A and suspension, which is applied in emulsion delivery, neurological diseases, liquid delivery, etc., and can solve problems such as waste, inconvenient use, and inability to guarantee needle penetration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
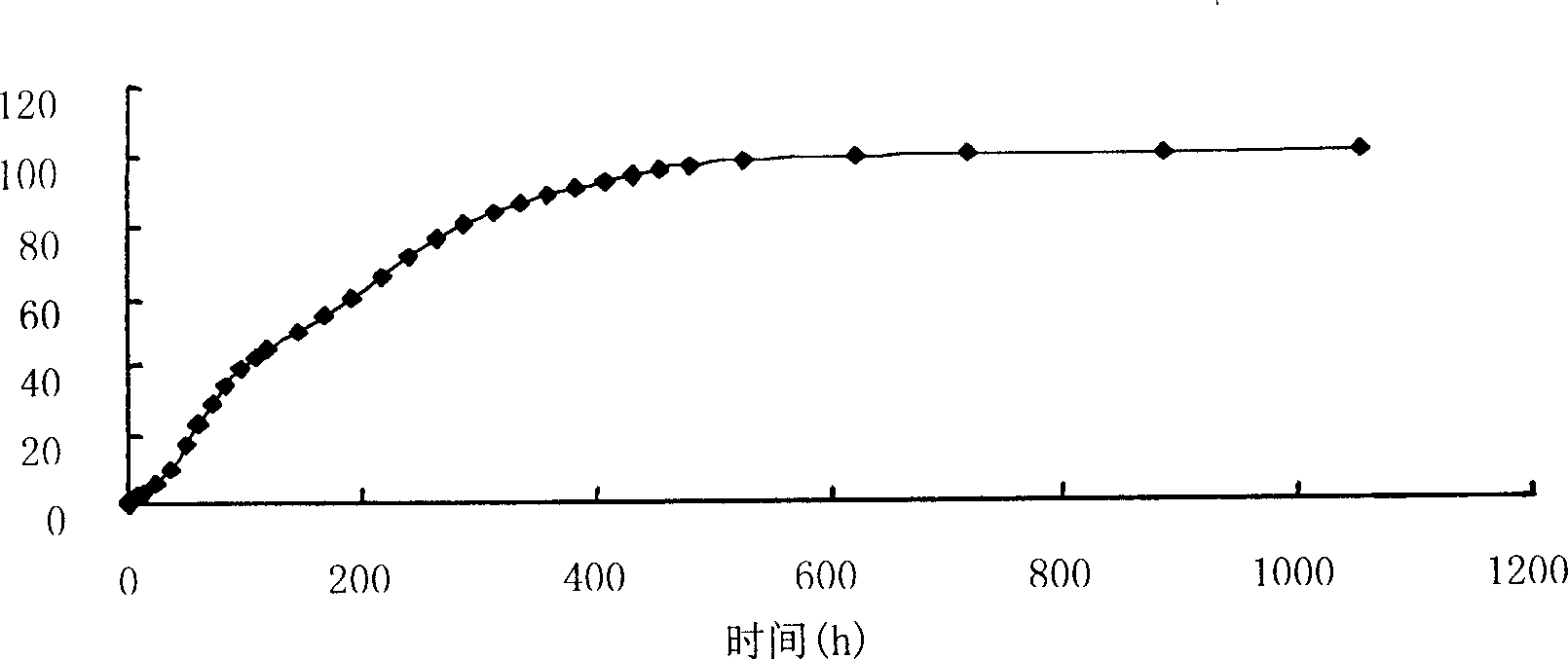
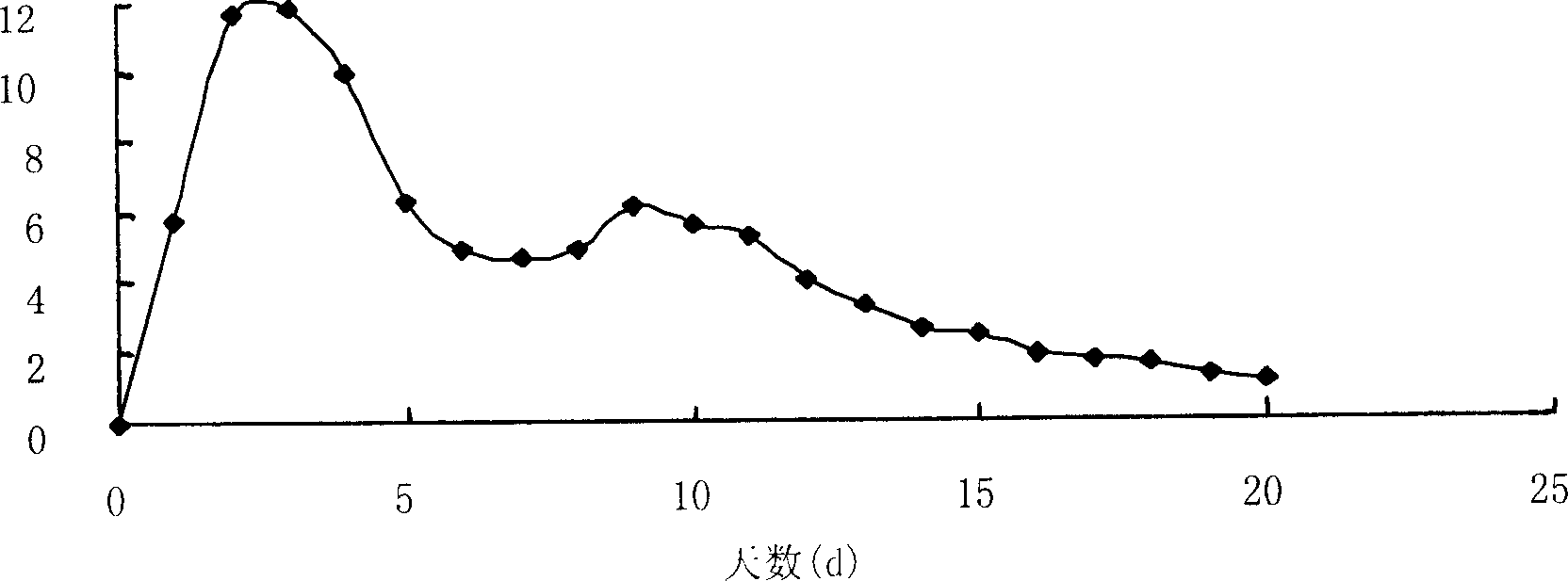
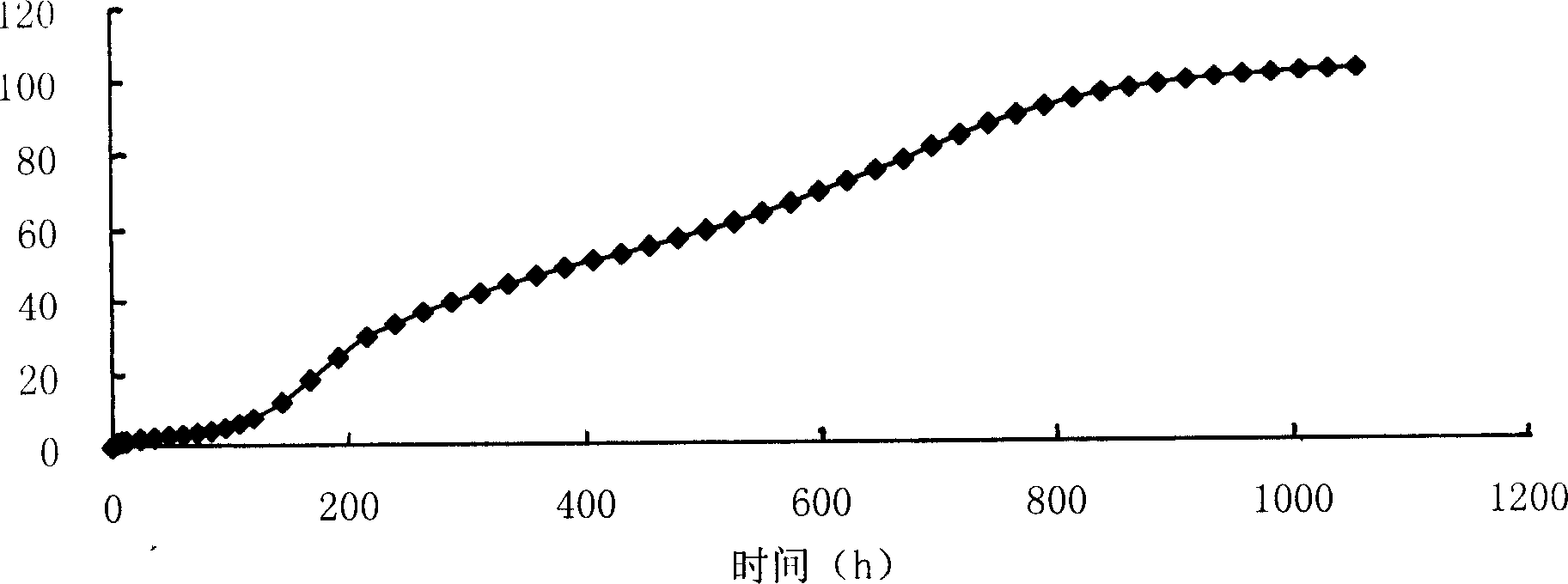
[0034]Weigh 40 mg of huperzine A, 400 mg of PLGA-COOH (75 / 25) in a small beaker, add 1 mL of dichloromethane (DCM) to dissolve them, and inject the drug-PLGA-DCM mixed solution with a syringe under mechanical stirring In 100mL aqueous solution containing 2% polyvinyl alcohol, fully emulsify for 30 minutes, reduce the stirring speed, collect microspheres after the organic solvent evaporates, wash with water, and dry. The whole operation process adopts aseptic operation. The drug loading of the obtained microspheres was 5.04%.
Embodiment 2
[0036] Weigh 30 mg of huperzine A, 100 mg of PLGA-COOH (75 / 25) in a small beaker, add 1 mL of dichloromethane (DCM) to dissolve them, and inject the drug-PLGA-DCM mixed solution into 10 mL with a syringe under magnetic stirring In an aqueous solution containing 1% polyvinyl alcohol, after fully emulsifying for 30 minutes, reduce the stirring speed, collect the microspheres after the organic solvent is evaporated, wash with water, and dry, and sterilize the obtained microspheres with ethylene oxide at 30°C for 48 hours to ensure The residual amount of ethylene oxide is below 5ppm. The drug loading of the obtained microspheres was 5.96%.
Embodiment 3
[0038] Weigh 20 mg of huperzine A, 100 mg of PLGA-COOH (75 / 25) in a small beaker, add 1 mL of dichloromethane (DCM) to dissolve them, and inject the drug-PLGA-DCM mixed solution into 10 mL with a syringe under magnetic stirring In an aqueous solution containing 1% polyvinyl alcohol, after fully emulsifying for 30 minutes, reduce the stirring speed, collect the microspheres after the organic solvent is evaporated, wash with water, and dry, and sterilize the obtained microspheres with ethylene oxide at 30°C for 48 hours to ensure The residual amount of ethylene oxide is below 5ppm. The drug loading of the obtained microspheres was 4.39%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com