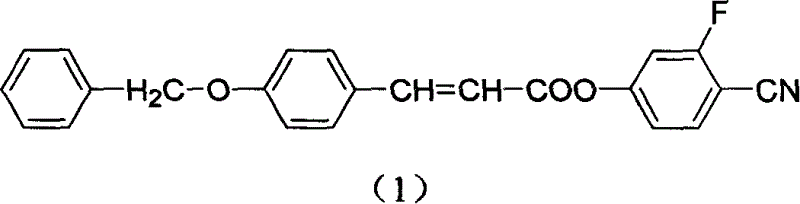
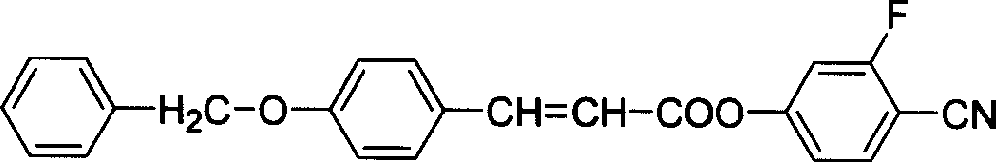
Novel LCD compound p-phenyl-methoxy cinnamic acid -2-fluoro-4-hydroxy- benzonitrile ester and its preparation method
A technology of benzyloxyl and liquid crystal compounds, which is applied in the field of organic synthesis, can solve problems such as low working voltage and low viscosity, and achieve the effects of high clearing point, stable chemical properties, and wide range of liquid crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
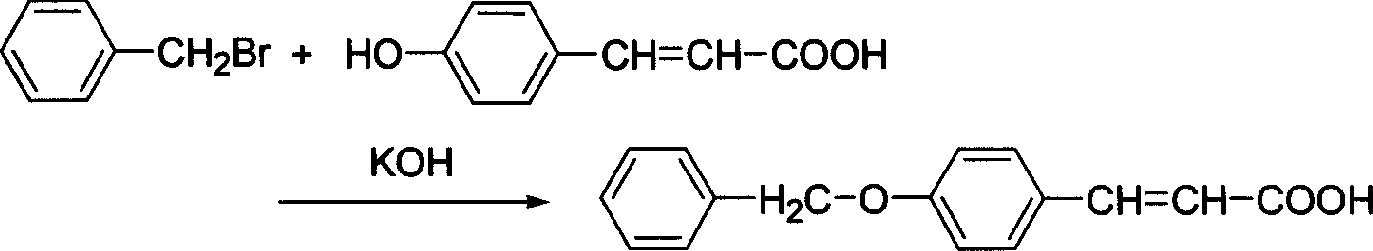
[0015] 1. Dissolve 0.01mol p-hydroxycinnamic acid and 0.02mol potassium hydroxide in 40ml 95% ethanol, then add 0.03mol bromotoluene dropwise at a rate of about 1ml / min, reflux for 24 hours, then add 0.02mol potassium hydroxide 14ml of 70% (volume fraction) ethanol solution, and continue to reflux for 2h. Then add 100ml of water, 20ml of concentrated hydrochloric acid, after heating for 15min, cool and filter, wash with distilled water, dry, and then recrystallize with 95% ethanol to obtain 4-benzyloxycinnamic acid as white flaky crystals with a yield of 54 %.
[0016] 2. Add 0.01mol of 4-p-benzyloxycinnamic acid and 20ml of anhydrous tetrahydrofuran into a three-necked flask, stir to dissolve it completely, then add 0.01mol of DCC (N,N-dicyclohexylcarbodiimide), After turbidity appears, dissolve 0.01mol of 2-fluoro-4-hydroxybenzonitrile and a small amount of DMAP[4-(N,N-dimethylamino)pyridine] in 20ml of anhydrous tetrahydrofuran to form a solution, and slowly drop it into t...
Embodiment 2
[0018] 1. Dissolve 0.02mol p-hydroxycinnamic acid and 0.05mol potassium hydroxide in 100ml 95% ethanol, then add 0.03mol bromotoluene, reflux reaction, and check the progress of the reaction by thin-layer chromatography until the raw materials disappear. Then add 35ml of 70% (volume fraction) ethanol solution of 0.03mol potassium hydroxide, and continue to reflux for 2h. Add 100ml of water and 40ml of concentrated hydrochloric acid, mix well, heat for 20min, cool and filter, wash with distilled water, dry, and then recrystallize with 95% ethanol to obtain 4-benzyloxycinnamic acid as white flaky crystals, the yield 49%.
[0019] 2. Add 0.015mol of 4-p-benzyloxycinnamic acid and 50ml of anhydrous tetrahydrofuran into a three-necked flask, stir to dissolve completely, then add 0.01mol of DCC (N,N-dicyclohexylcarbodiimide), After turbidity appears, completely dissolve 0.015mol 2-fluoro-4-hydroxybenzonitrile and a small amount of DMAP[4-(N,N-dimethylamino)pyridine] in 35ml of anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com