New pirkle-type chiral fixed phase and its prepn process
A chiral stationary phase and reaction technology, applied in the field of chiral separation, to achieve the effect of simple reaction route, easy industrialization, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
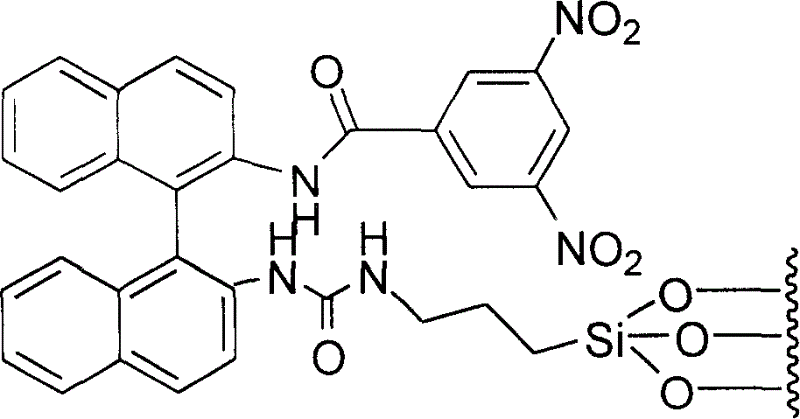
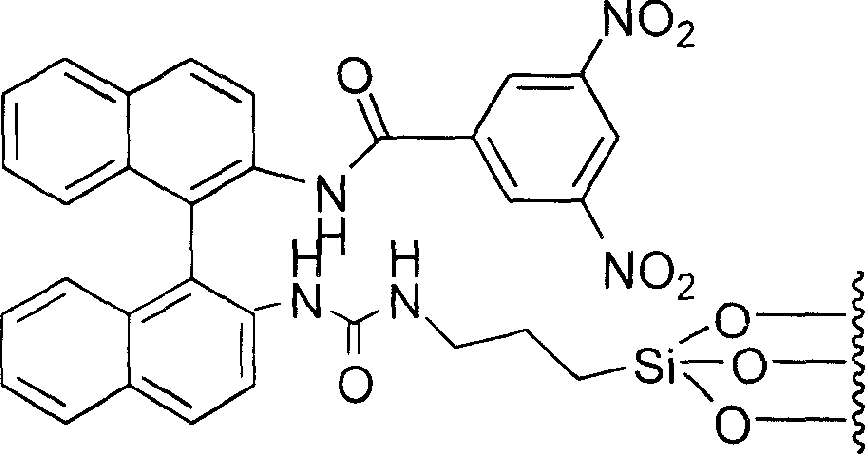
[0018] Dissolve 1.21g (4.26mmol) of R-(+)-1,1'-binaphthyl-2,2'-diamine in 100ml of dichloromethane, add 0.59ml (4.26mmol) of triethylamine, and mix The solution was placed in a 250ml double-necked round-bottomed flask; 0.98g (4.26mmol) of 3,5-dinitrobenzoyl chloride (DNB-Cl) was dissolved in 80ml of dichloromethane, and the solution was dissolved at room temperature under nitrogen protection. Drop it into the above stirring mixed solution as slowly as possible. After dropping, the resulting mixture was heated to reflux for 10 hours. After cooling, the reaction mixture was washed with 2mol / L hydrochloric acid solution (100ml×3), the organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated to dryness. The product was separated by silica gel column chromatography to obtain 1.79g of a red powdery solid. 1 Confirmed by H-NMR as N-[2'-amino-[1,1']binaphthyl-2-]-3,5-dinitrobenzamide, yield: 88%.
[0019] Dissolve 1.79g (3.74mmol) of it in 100ml of di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com