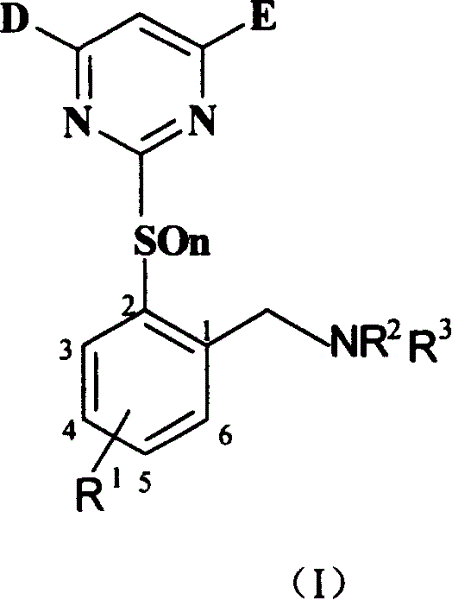
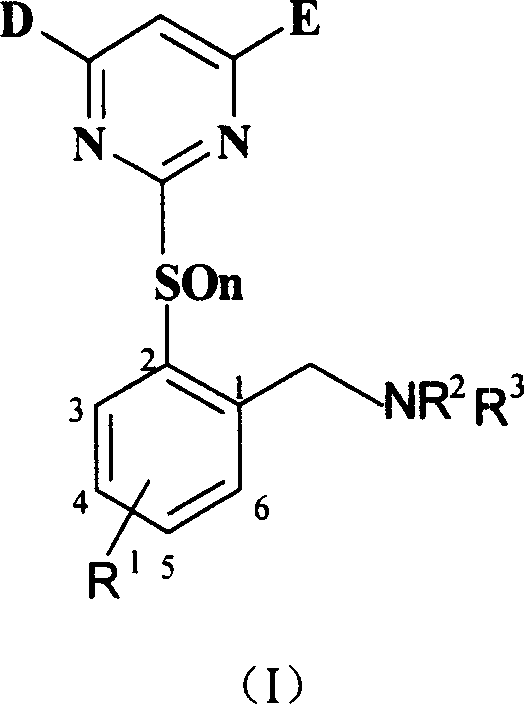
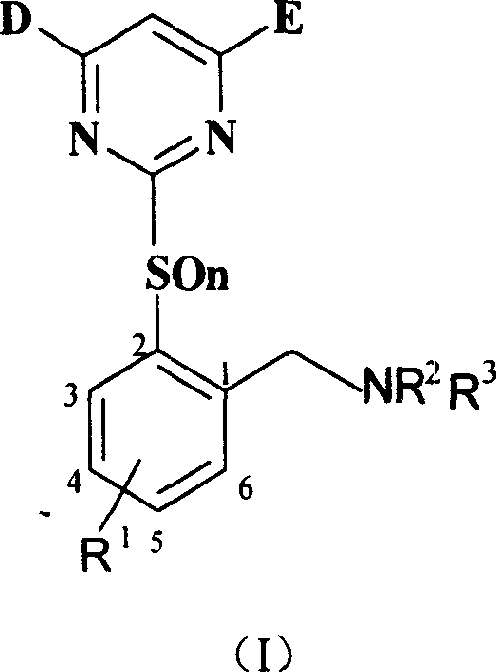
Benzylamine compound, and its preparing method and use
A kind of technology of benzylamines and derivatives, applied in the field of new benzylamine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of intermediate 2-methyl-3-chlorothiophenol (V):
[0048] Add 80ml concentrated hydrochloric acid to the flask, ice bath, add 64.5g (0.4mol) 2-methyl-3-chloroaniline under mechanical stirring, it takes about half an hour, then stir for half an hour, keep 0℃ and add 34g (0.4mol) dropwise The solution of sodium nitrite and 68ml water takes about half an hour. Stir at 0℃ for 1 hour to obtain the diazonium salt solution. Add 64g (0.4mol) potassium ethanoate and 100ml water in a 1000ml flask, stir and heat to 40 -45℃, keep the temperature, drop in the above diazonium salt solution for about 2 hours, stir for 1 hour after the addition, cool, separate the lower oily substance, extract the water layer with ether, evaporate the ether, add 200mL anhydrous Ethanol, add 90 grams (1.6 mol) of potassium hydroxide in batches. The addition speed is appropriate to maintain a slight boiling. After the addition, heat to reflux for 6 hours, cool and reduce the pressure to evaporate t...
Embodiment 2
[0050] Preparation of intermediate 2-(4.6-dimethoxy-2-pyrimidinyl mercapto)-6 chlorotoluene (VI-1):
[0051] Add 15.8 grams of 2-methyl-3-chlorothiophenol (0.1 mol), 13.8 grams of anhydrous potassium carbonate (0.1 mol), 21.8 grams (0.1 mol) of 4.6-dimethoxy-2-methane sulfonate into a 250 mL flask Acylpyrimidine and 200mL tetrahydrofuran, reflux for 4 hours, cool, filter with suction, wash a small amount of tetrahydrofuran, evaporate the tetrahydrofuran, add 100mL methanol, stir to precipitate a white solid, filter and dry, get 18.9 g, then concentrate and cool to crystallize to get 3.6 g, yield 76%.
Embodiment 3
[0053] Preparation of intermediate 2-(4.6-dimethoxy-2-pyrimidinyl mercapto)-6-chlorobenzyl bromide (VII-1):
[0054] Add 5.93 grams (20m mol) of 2-(3,5-dimethoxypyrimidinylmercapto)-6-chlorotoluene, 3.63 grams (20m mol) of N-bromosuccinimide, 0.3g Nitrogen diisobutyronitrile, dry CCl 4 100mL, connected to reflux condenser and drying tube, heated and refluxed for 4 hours, cooled, filtered, and dissolved under reduced pressure. 15mL of methanol was added and stirred at room temperature. A white solid was precipitated, filtered and dried to obtain 5.9 g. The yield was 78.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com