Production of LixCoO2 from recovering waste lithium ionic battery
A technology of lithium-ion batteries and ions, which is applied in the field of chemical separation and preparation of inorganic powders, and can solve problems such as harsh operating environments, atmospheric pollution, and environmental hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Take various types of lithium-ion batteries, cut the battery in water, separate the positive electrode material from other components, weigh 150g of the positive plate, cut the positive plate into pieces smaller than 2cm×2cm, and put these pieces at room temperature Put into 300ml of N-methyl-2-pyrrolidone (NMP) liquid, heat the liquid to 85°C, and after 30 minutes, all the powder substances (including positive electrode active material Li x CoO 2 and carbon black) fall off into the liquid, take out the aluminum foil, filter the liquid and reclaim NMP (recyclable), wash the filtered powder with clear water, dry to obtain 127g (theoretical value should be 128.7g) black powder; The black powder was put into 1.4L concentration of 15% (wt.) nitric acid solution, and while stirring, was added dropwise to the solution with a concentration of 5% (wt.) H 2 o 2 Solution 0.18L, continue stirring for about 40 minutes, filter to obtain 2+ , Li + The nitrate solution of ion, the...
example 2
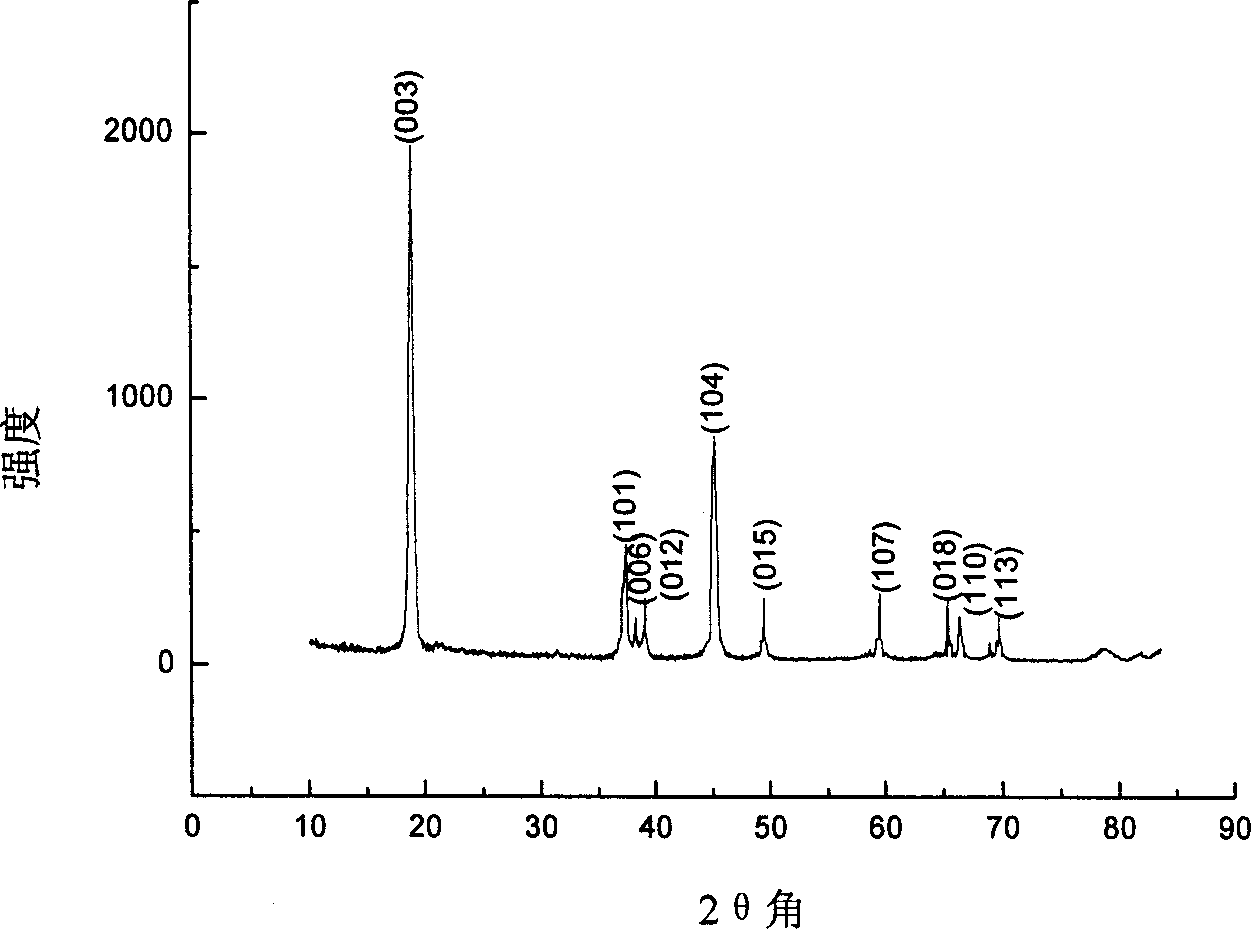
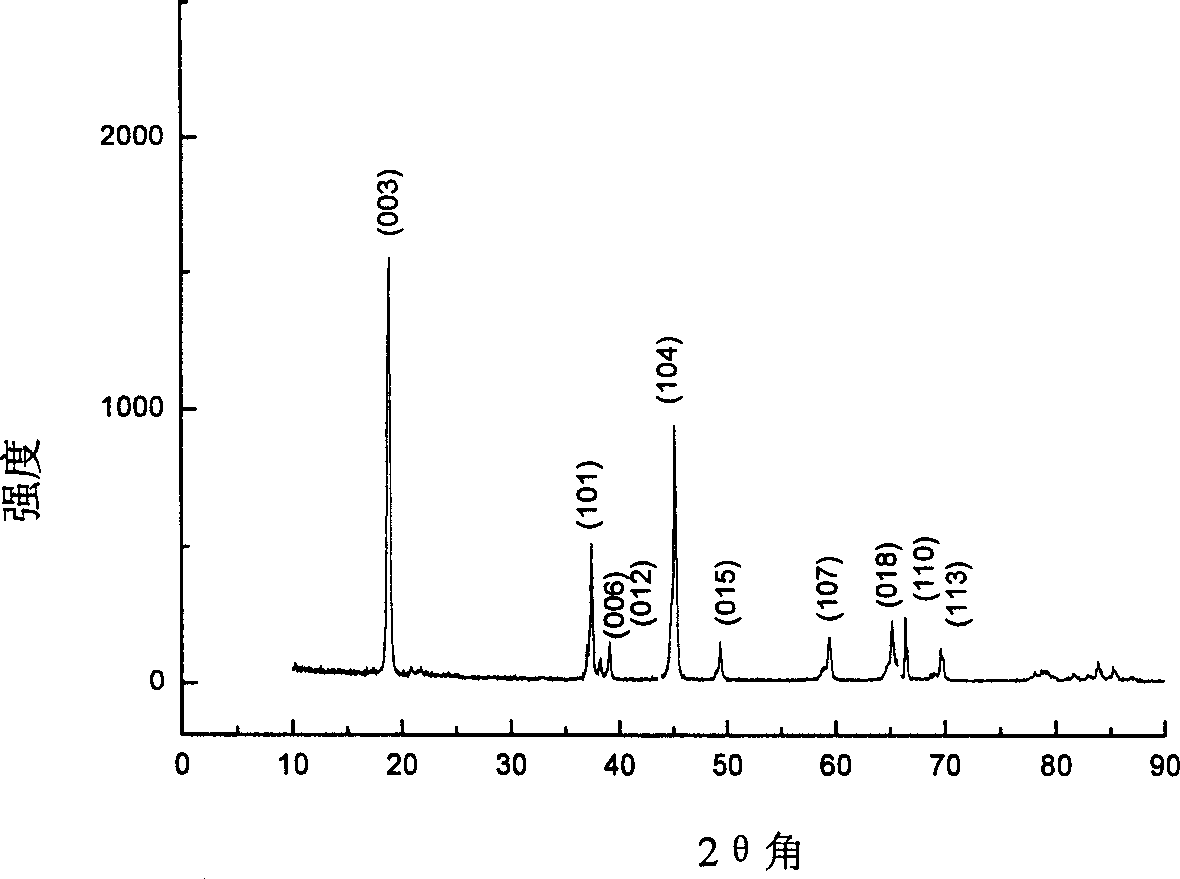
[0023] The precursor of the synthesized product was prepared by the same process as in Example 1, and the precursor was calcined at a temperature of 800 ° C for 8 hours to obtain Li with a grain size of less than 1 μm x CoO 2 Powder. The crystal phase analysis of the obtained powder is as follows: figure 2 As shown in the X-ray diffraction pattern of the powder, the lattice parameters of the powder are shown in Table 2, indicating that the prepared Li x CoO 2 The powder has a regular hexagonal layered structure; the specific surface area of the powder of sample 1 measured by the nitrogen BET multilayer adsorption method is 5.12m 2 g -1 ; The prepared Li x CoO 2The primary discharge capacity is 147mAh·g -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com