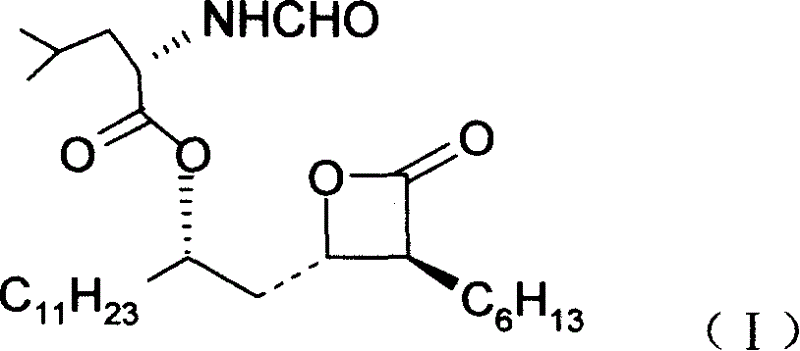
Orlistat preparation method
An orlistat and compound technology, applied in the field of pharmaceutical preparation, can solve the problems of increasing the production cost of β-hydroxyester, low economic and practical value, long synthesis route, etc., and achieve high practical value, low price, and reduced production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
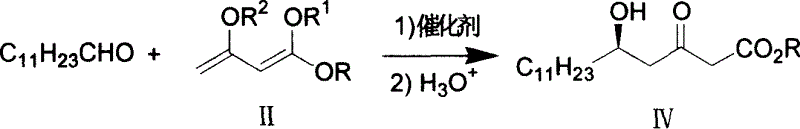
[0043] The first step of (5R)-5-hydroxy-3-oxocetanoic acid methyl ester (IV) preparation
[0044] A solution of (S)-(-)-BINOL (458 mg, 1.6 mmol) in THF was added to Ti(OPr-i) under nitrogen 4 (455mg, 1.6mmol). The solution was stirred at room temperature for 20 minutes, then lauraldehyde (14.75g, 80mmol), lithium chloride (1.10g, 0.026mol) and N,N,N',N'-tetramethylethylenediamine (6.14g , 0.053mol) was added. The mixture was stirred at room temperature for 20 minutes, and then 1,3-bis(trimethylsilyloxy)-1-methoxy-1,3-butadiene (48.7g, 187mmol) was added dropwise. Stir for 4 hours. Add NaHCO after the reaction 3 aqueous solution, and the organic layer was treated with ethyl acetate to obtain compound formula (IV).
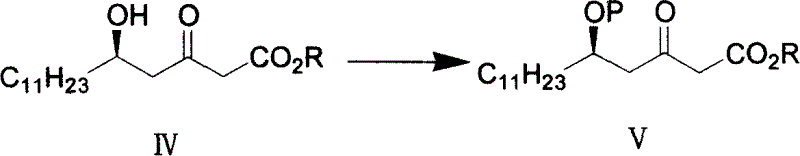
[0045] The second step (5R)-5-p-methoxybenzyloxy-3-oxocetanoic acid methyl ester, prepared as formula (V)
[0046] A solution of 4-methoxybenzyl alcohol (5.18 g, 37.5 mmol) in ether was added to NaH (90 mg, 3.75 mmol, 60% mineral / oil) in ether at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com