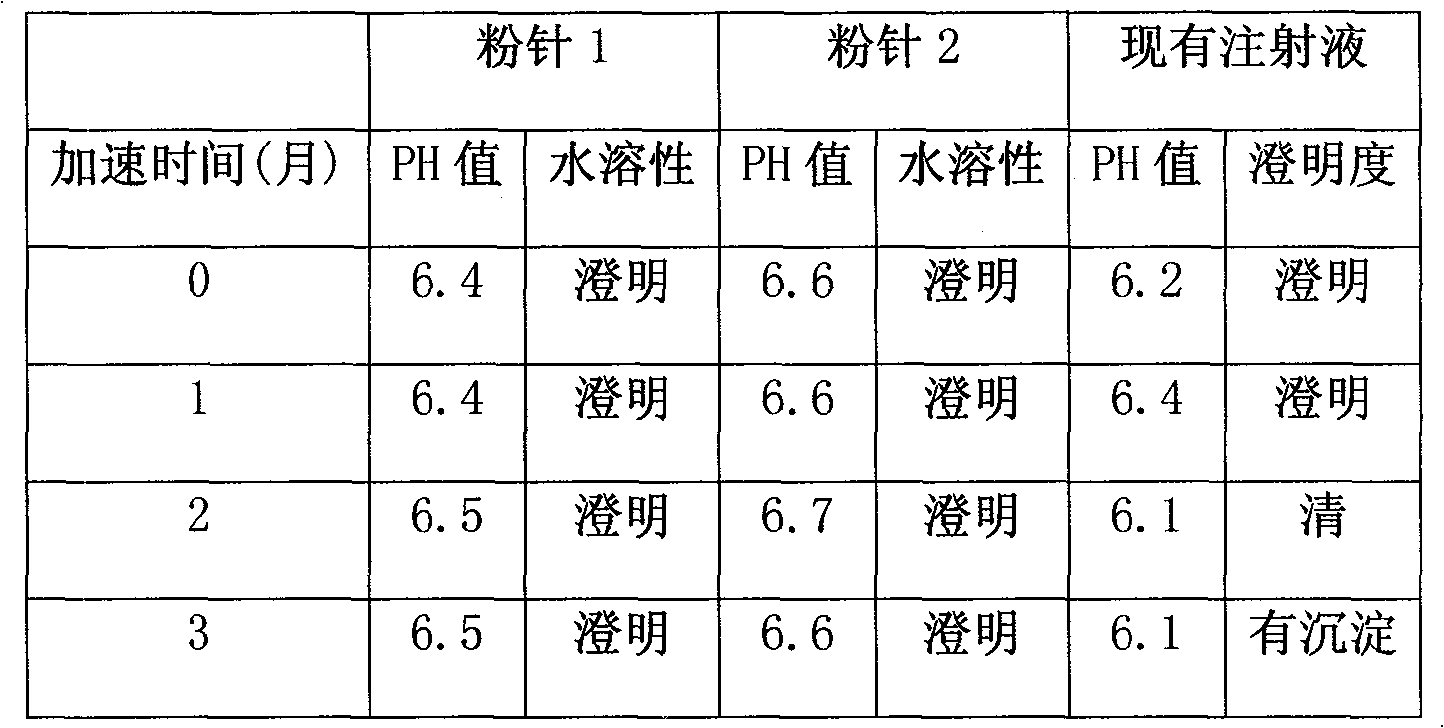
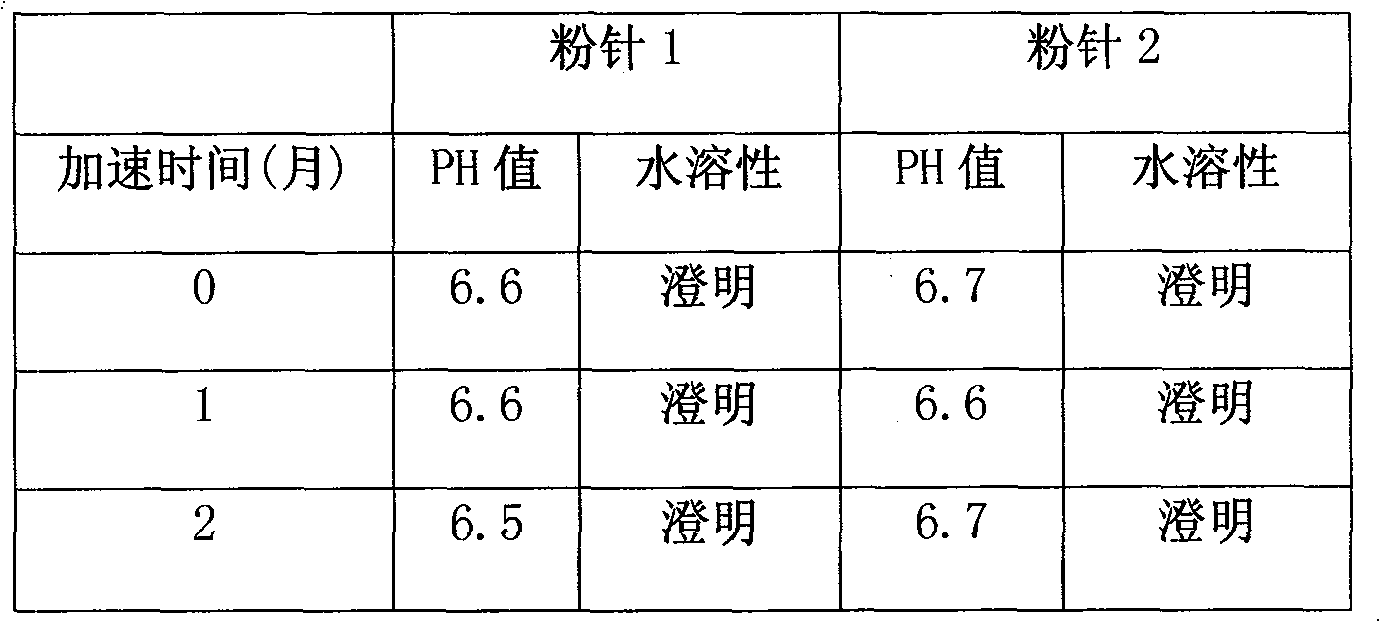
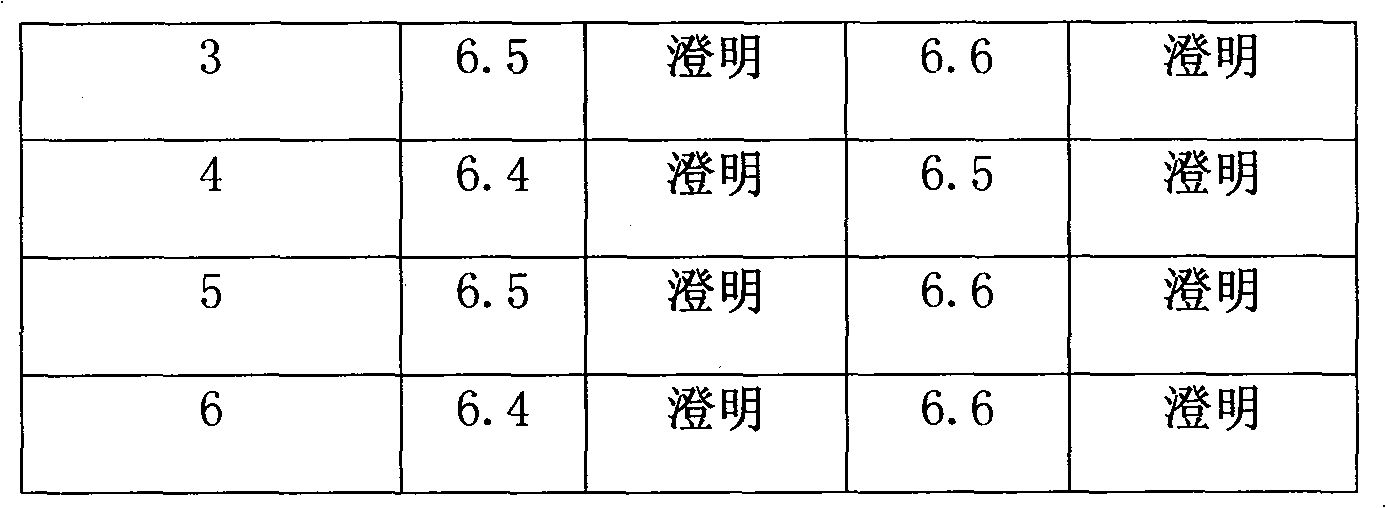
Powder injection containing red-rooted salvia
A kind of technology of Salvia miltiorrhiza and injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The raw materials used Danshen 375g, Panax notoginseng 118g, Astragalus 100g, and Dalbergia oil 8g.
[0081] Add 0.5% hydroxypropyl-β-cyclodextrin aqueous solution to the dalbergia oil, stir to dissolve, and filter to prepare an inclusion compound solution. Extract Astragalus, Danshen and Panax notoginseng with ethanol to obtain the ethanol extract, filter the extract with gauze, collect the filtrate, concentrate the filtrate to obtain an extract with a relative density of 1.32~1.40 (55℃), add the above inclusion compound solution , Then add 49g of mannitol, add water to dissolve, coarsely filter, and the filtrate is ultrafiltered; the ultrafiltrate is pre-frozen to -40°C, kept for 3 hours, evacuated to a pressure of 13Pa and maintained in the vacuum state, within 20 hours, the temperature Raise the temperature to -7°C, then raise the temperature to 30°C within 5 hours, and keep it for 3 hours. Dispense the dry powder into sterile injection bottles under aseptic conditions...
Embodiment 2
[0084] The raw materials used Danshen 465g, Panax notoginseng 30g, Astragalus 85g, and Dalbergia odorifera 8g.
[0085] 0.5% β-cyclodextrin aqueous solution was added to the dalbergia oil, stirred to dissolve, and filtered to prepare an inclusion compound solution. Add 5 times the amount of water to the coarsely crushed Danshen and Astragalus, decoct for 2 hours, filter, and extract the filter residue for the second time. Add 4 times the amount of water, decoct for 1 hour, filter, discard the filter residue, combine the filtrate to obtain Danshen Astragalus extract. The Panax notoginseng is extracted with 70% ethanol to obtain the Panax notoginseng extract. Combine the above Danshen and Astragalus extract and Panax notoginseng extract, stand still, filter, and concentrate the filtrate to obtain an extract with a relative density of 1.32~1.40 (55℃). Add the above inclusion compound solution, then add mannitol i8g, add water Dissolve, coarsely filter, and ultrafilter the filtrate...
Embodiment 3
[0088] The raw materials used Danshen 260g, Panax notoginseng 165g, Astragalus 170g, and Dalbergia oil 8g.
[0089] Add 0.5% hydroxypropyl-β-cyclodextrin aqueous solution to the dalbergia oil, stir to dissolve, and filter to prepare an inclusion compound solution. Using 80% ethanol to extract Danshen, Panax notoginseng and Astragalus to obtain a mixed ethanol extract, the extract was centrifuged at a high speed and the supernatant was separated, and the supernatant was concentrated to obtain an extract with a relative density of 1.32~1.40 (55℃). Add the above inclusion compound solution, then add 50g of lactose, add water to dissolve, coarsely filter, and the filtrate is ultrafiltered; the ultrafiltrate is pre-frozen to -35°C, kept for 2 hours, evacuated to a pressure of 15Pa and maintained in the vacuum state, 25 Within hours, increase the temperature to -10°C, and then within 5 hours, increase the temperature to 20°C, and keep it for 5 hours. Dispense the dry powder into steril...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com