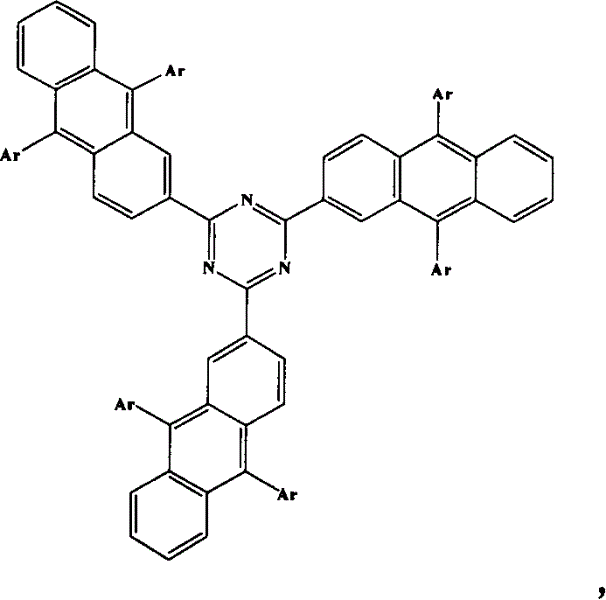
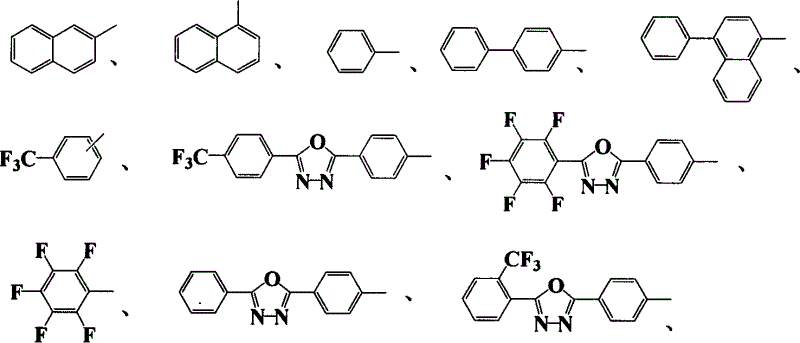
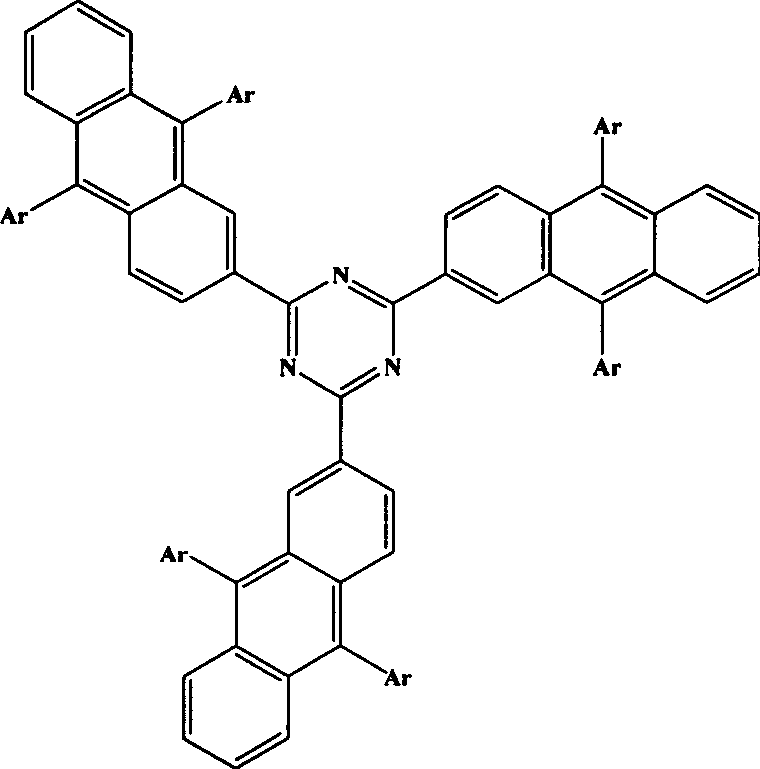
Novel blue light material-thiotrzinone-containing anthracene derivatives
A technology of triazine rings and derivatives, which is applied in the field of new anthracene derivatives containing triazine rings, can solve the problems of industrialization and other problems, and achieve good electron transfer performance, high thermal stability, and excellent electrochemical performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Synthesis of 2-methyl-9,10-diphenyl-anthracene
[0029]
[0030] Under argon protection and vigorous stirring, a solution of bromobenzene (7.9g, 50mmol) in THF (tetrahydrofuran, 40mL) was added dropwise at 60°C to a solution containing magnesium (1.44g, 60mmol), a catalytic amount of iodine and THF (40mL). In the mixed solution, after dropping, continue to stir and reflux for 3 hours, then cool to room temperature. The resulting solution was added dropwise to a THF (80mL) solution of 2-methylanthraquinone (5.4g, 24mmol) cooled to -78°C under the protection of argon under the protection of argon. After the addition was complete, the reaction solution was naturally warmed to room temperature and stirred. After overnight, pour into 100 mL of distilled water and extract twice with dichloromethane (200 mL). The organic phases were combined, washed with water, washed with saturated brine and dried over anhydrous sodium sulfate, then filtered, and the solvent was...
Embodiment 2
[0031] Example 2 Synthesis of 2-methyl-9,10-bis(2-naphthyl)-anthracene
[0032]
[0033] In an atmosphere of argon, 2-bromonaphthalene (10.4 g, 50 mmol) was added dropwise to a vigorously stirred mixture containing magnesium (1.4 g, 60 mmol), catalytic amount of iodine and THF (40 mL) at 60 °C solution in THF (40 mL). After dropping, the reaction solution was stirred and refluxed for 3 hours, then cooled to room temperature. The resulting solution was added dropwise to a solution of 2-methylanthraquinone (5.4 g, 24 mmol) in THF (80 mL) that had been cooled to -78°C under the protection of argon with stirring. After the addition was complete, the reaction solution was naturally warmed to room temperature and stirred overnight, poured into 100mL distilled water, extracted twice with dichloromethane (200mL), combined the organic phases, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate and filtered, and the filtrate was distilled off the solv...
Embodiment 3
[0034] Example 3 Synthesis of 2-methyl-9,10-bis(pentafluorophenyl)-anthracene
[0035]
[0036] In an argon atmosphere, at -78°C, in a solution of 1-bromo-2,3,4,5,6-tetrafluorobenzene (12.4g, 50mmol) in THF (40mL), slowly dropwise add n-butyllithium n-Hexane solution (1.6 mol / L, 35 mL). After stirring at this temperature for 1 hour, the cooling bath was removed and the reaction mixture was allowed to warm to room temperature naturally. The obtained material was slowly added to a solution of 2-methylanthraquinone (5.4 g, 24 mmol) in THF (80 mL) which had been cooled to -78°C under the protection of argon. After the addition was complete, the reaction solution was naturally warmed to room temperature, then heated to reflux, and stirred overnight at this temperature. After the mixture was cooled to room temperature, it was poured into 100 mL of distilled water, and extracted twice with dichloromethane (200 mL). The organic phases were combined, washed with water, washed wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com