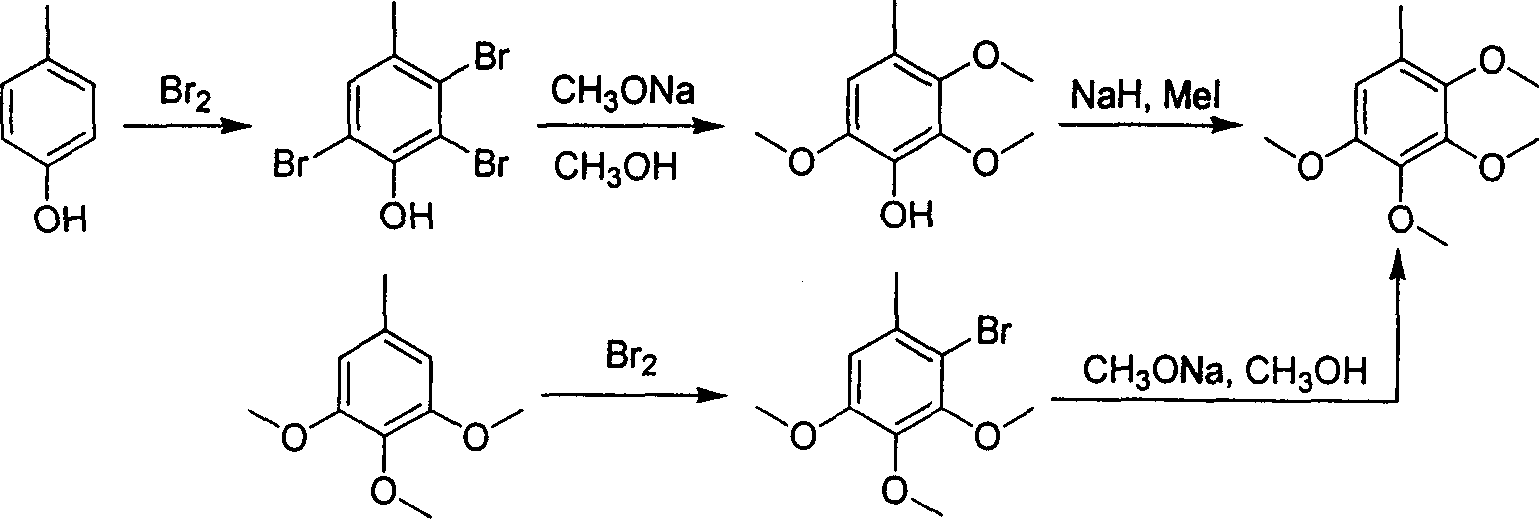
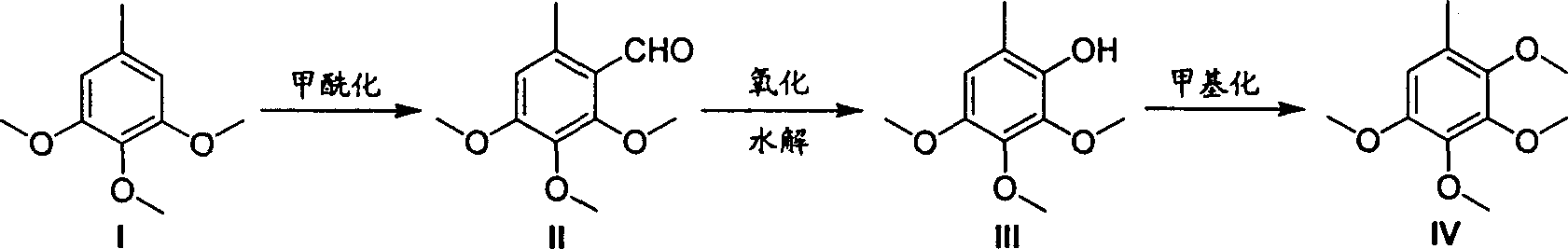
2,3,4,5-tetramethoxyl toluene synthesis method
A technology of tetramethoxytoluene and a synthesis method, which is applied in the field of chemical synthesis technology, can solve the problems of inconvenient operation, low yield, difficult product purification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 91 grams of 3,4,5-trimethoxytoluene in 300 milliliters of DMF, add 250 grams of phosphorus oxychloride dropwise under cooling in a water bath, and control the temperature in the reactor below 50°C. React at 90°C until the raw materials are completely reacted. After cooling to room temperature, add ice-water mixture to the reaction kettle, neutralize to neutral with sodium hydroxide solution, then cool to room temperature, precipitate crystals, filter and wash with water. Vacuum drying gave 90.5 g of 2,3,4-trimethoxy-6-methylbenzaldehyde with a yield of 86.2%.
Embodiment 2
[0025] Add 500 milliliters of ethanol and 200 grams of m-chloroperoxybenzoic acid to the reaction kettle, dissolve 90.5 grams of 2,3,4-trimethoxy-6-methylbenzaldehyde in ethanol, add dropwise to the reaction kettle, and add dropwise After finishing, continue to react until raw material reaction is complete. Sodium thiosulfate solution was added, the aqueous phase was extracted with ethyl acetate, dried and then concentrated to obtain 81.3 g of 2,3,4-trimethoxy-6-methylphenol with a yield of 95.3%.
Embodiment 3
[0027] 81.3 g of 2,3,4-trimethoxy-6-methylphenol was dissolved in 500 ml of tetrahydrofuran solvent, 35 g of 60% sodium hydride was added, and 150 g of methyl iodide in tetrahydrofuran (200 ml) was added dropwise. After the dropwise addition, the reaction was refluxed until the reaction of the raw materials was complete, 500 ml of ethyl acetate was added, washed with water, dried and concentrated to obtain crude 2,3,4,5-tetramethoxytoluene. Fractions at 113-115° C. / 3 mmHg were collected by vacuum distillation to obtain 79 grams of pure 2,3,4,5-tetramethoxytoluene with a yield of 90.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com