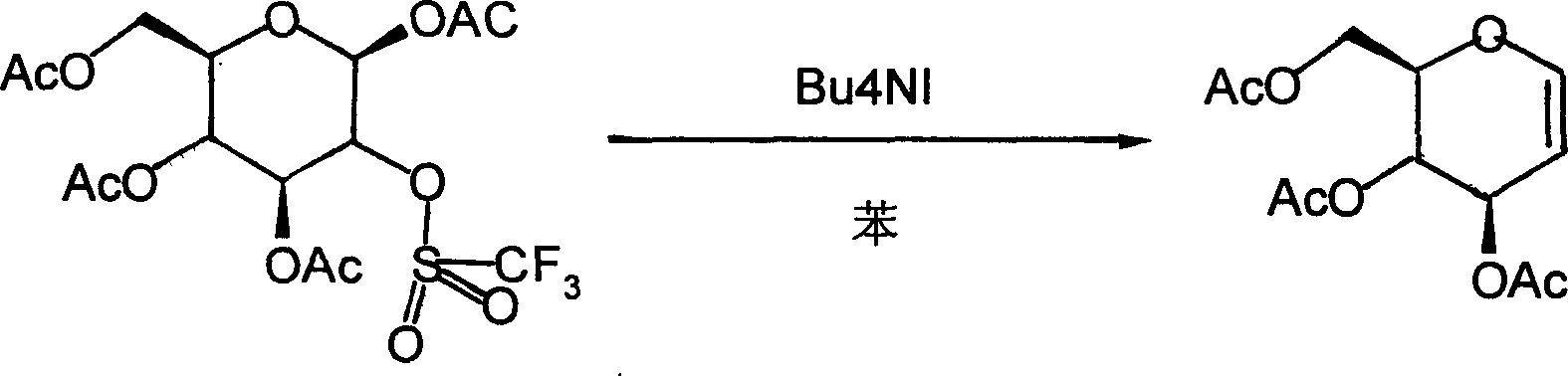
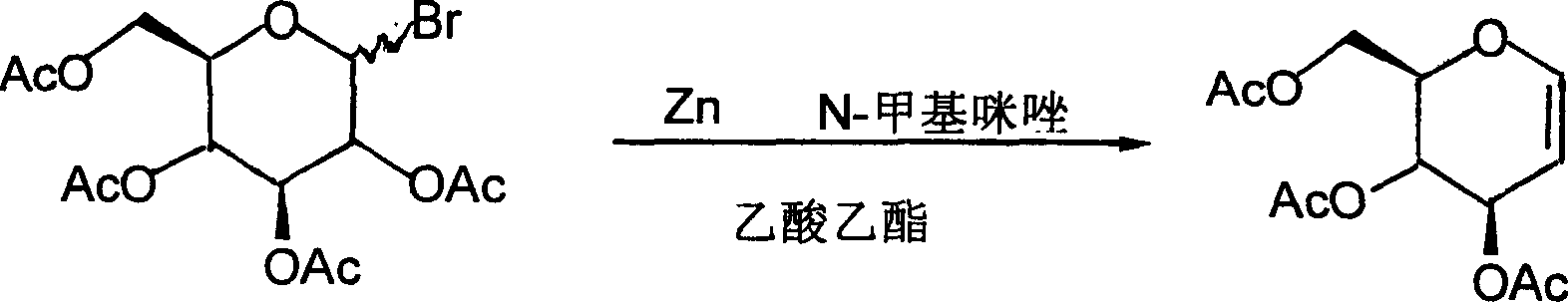
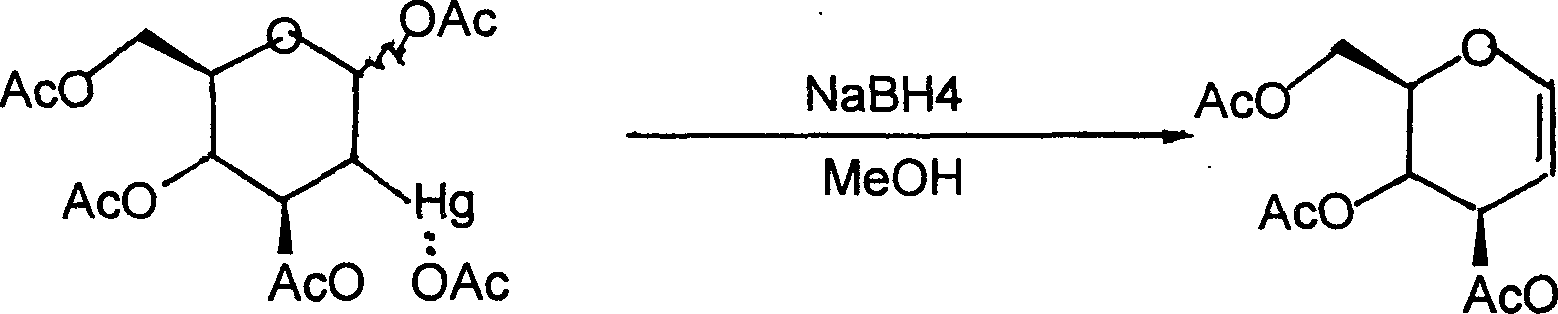Method for preparing acetylated glucal
A technology of acetylated glucose and acetylation, applied in the field of preparation of acetylated gluculose, can solve the problems of rare reagents, cumbersome post-processing and high cost, and achieve the effect of reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Example 1: Add 55kg D-glucose, 200L acetic anhydride, and catalytic amount of perchloric acid (10ml) to a 500L reactor, stir and dissolve, and control the reaction temperature at 15-45°C for 2 hours. At the end of the heat preservation, cool to below 5°C, pass in 7 cubic meters of hydrogen bromide, control the reaction temperature at 0-10°C and react for 2 hours. After the reaction is completed, pour it into a 2000L reactor (with 1000L of water), and then add 200L of chloroform , stirred for 30min, separated the chloroform, extracted the water layer once more with 100L of chloroform, combined the chloroform, then added 100L saturated aqueous solution of sodium bicarbonate to the chloroform, stirred for 30min, separated the chloroform, then added 300L of chloroform to the chloroform Saturated aqueous solution of sodium, stirred for 30 minutes, separated chloroform, transferred to a 500L dry kettle, added 25KG of anhydrous sodium sulfate, after 2 hours, filtered into a 500...
example 2
[0038] Example 2: Add 55kg D-glucose, 200L acetic anhydride, and catalytic amount of zinc chloride (50g) to a 500L reactor, stir and dissolve, and control the reaction temperature at 15-45°C for 2 hours. At the end of the heat preservation, cool to below 5°C, pass in 7 cubic meters of hydrogen bromide, control the reaction temperature at 0-10°C and react for 2 hours. After the reaction is completed, pour it into a 2000L reactor (with 1000L of water), and then add 200L of chloroform , stirred for 30min, separated the chloroform, extracted the water layer with 100L chloroform again, combined the chloroform, then added 100L saturated aqueous solution of sodium bicarbonate to the chloroform, stirred for 30min, separated the chloroform, then added 300L sodium chloride to the chloroform of saturated aqueous solution, stirred for 30min, separated chloroform, transferred to a 500L dry kettle, added 25KG of anhydrous sodium sulfate, and after 2 hours, filtered it into a 500L distillatio...
example 3
[0040] Example 3: Add 55kg of D-glucose, 200L of acetic anhydride, and 15ml of catalytic amount of p-toluenesulfonic acid to a 500L reactor, stir and dissolve, and control the reaction temperature at 15-45°C for 2 hours. At the end of the heat preservation, cool to below 5°C, add 90KG of phosphorus tribromide, then add 18KG of water, control the reaction temperature at 0-10°C for 2 hours, after the reaction is over, pour it into a 2000L reactor (with 1000L of water) , then add 200L of chloroform, stir for 30min, separate the chloroform, extract the water layer once more with 100L of chloroform, combine the chloroform, then add 100L of saturated aqueous solution of sodium bicarbonate to the chloroform, stir for 30min, separate the chloroform, and then add to the chloroform Add 300L of saturated aqueous solution of sodium chloride, stir for 30min, separate the chloroform, transfer to a 500L drying kettle, add 25KG of anhydrous sodium sulfate, after 2 hours, press filter into a 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com