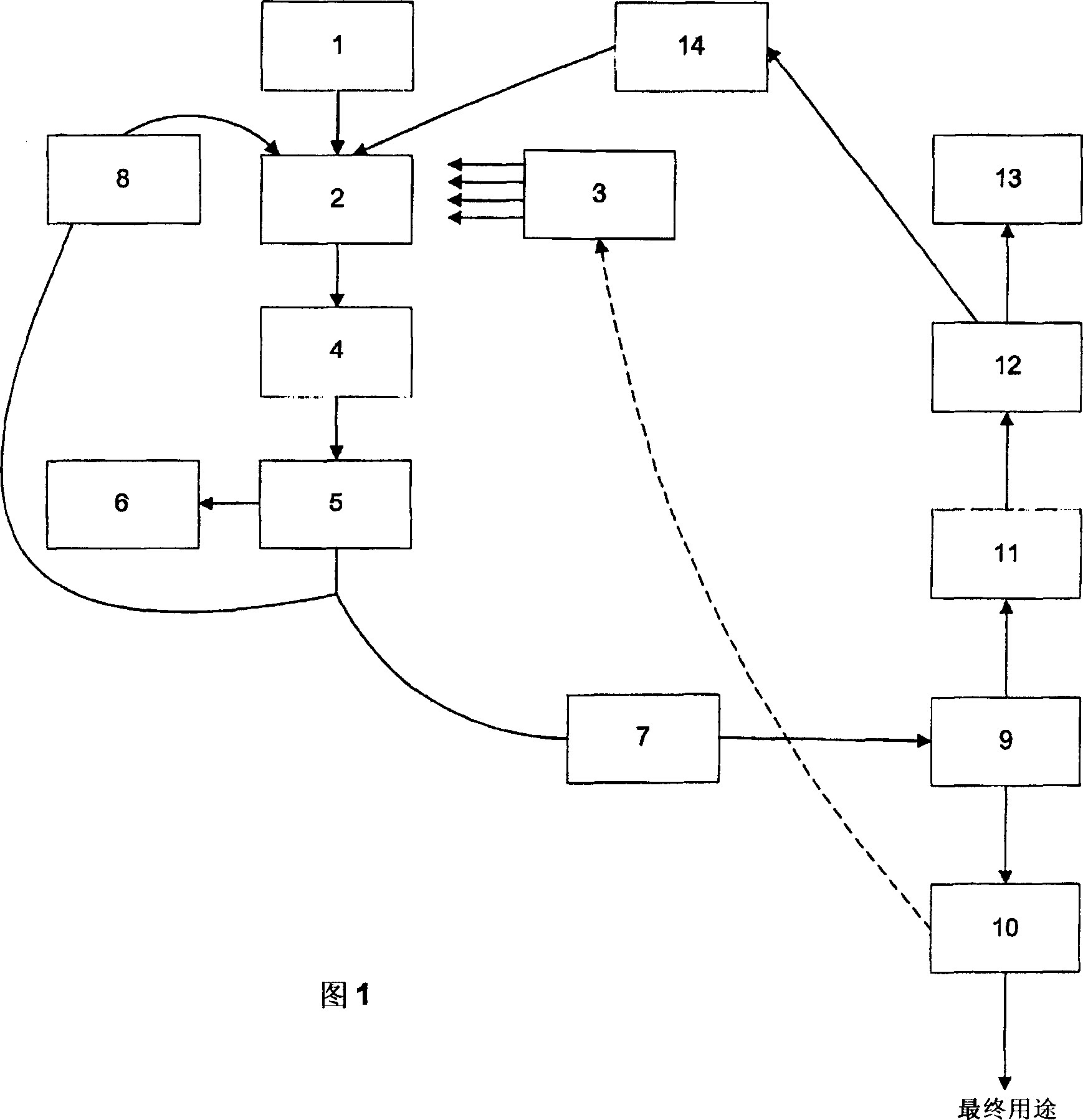
Solvents for use in the treatment of lignin-containing materials
A technology of lignin and lignocellulose, applied in the field of new chemical species, can solve the problems of high cost, difficulty in continuous implementation and complex design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0045] According to one embodiment, the ionic liquid comprises:
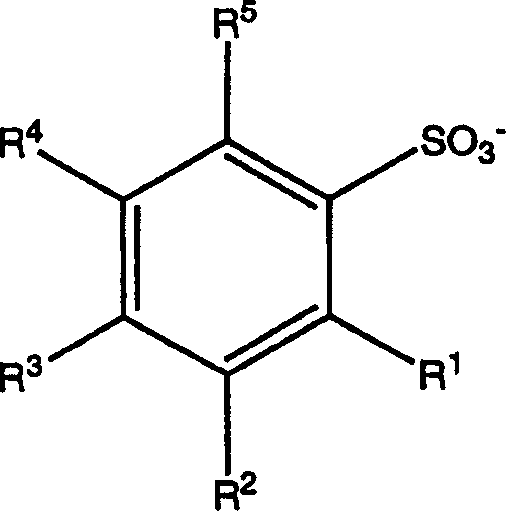
[0046] - substituted or unsubstituted aryl organic acid anions; and
[0047] - Inorganic or organic cations forming ionic liquids.
[0048] anion
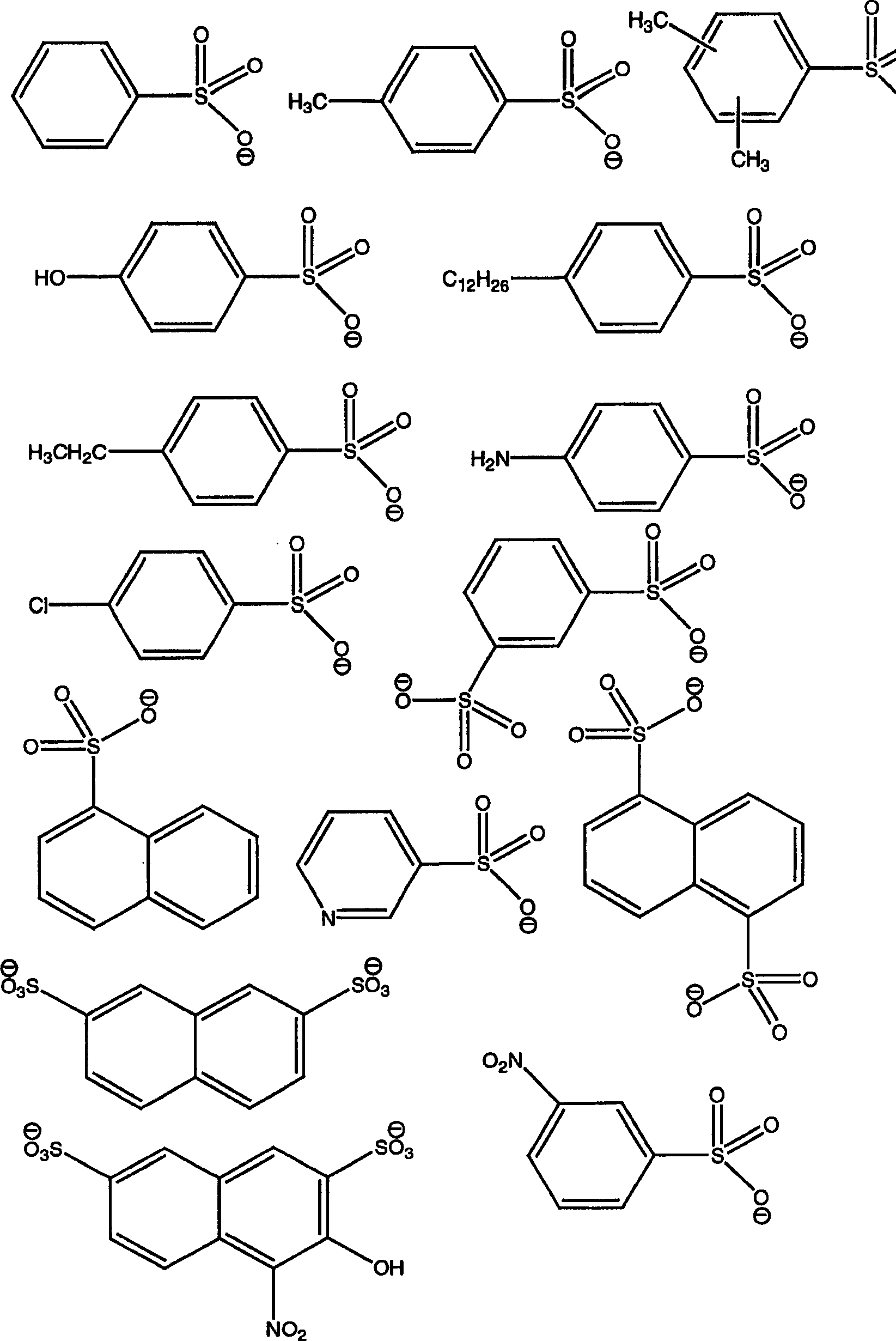
[0049] With regard to the anionic component, the term aryl refers to single, polynuclear, conjugated and / or fused residues of aromatic hydrocarbons or aromatic heterocyclic ring systems. Examples of aryl groups include phenyl, biphenyl, terphenyl, quaterphenyl, phenoxyphenyl, naphthyl, tetrahydronaphthyl, anthracenyl, dihydroanthracenyl, benzanthracenyl, dibenzo Anthracenyl, phenanthrenyl, fluorenyl, pyrenyl, indenyl, azulenyl, -yl, pyridyl, 4-phenylpyridyl, 3-phenylpyridyl, thienyl, furyl, pyrrolyl, pyrrolyl, furanyl , imidazolyl, pyrrolidinyl, pyridinyl, piperidino, indolyl, pyridazinyl, pyrazolyl, pyrazinyl, thiazolyl, pyrimidinyl, quinolinyl, isoquinolyl, benzofuryl , benzothienyl, purinyl, quinazolinyl, phenazinyl, acridinyl, benzoxazolyl, benzothiazolyl, et...
Embodiment 1
[0106] 1-Ethyl-3-methylimidazolium xylenesulfonate (ElmXS) was prepared by metathesis of sodium xylenesulfonate and 1-ethyl-3-methylimidazolium bromide.
[0107] EmImXS is a liquid salt that can be used to extract lignin from pulp at a temperature of about 150°C in a solvent mixture comprising water and a little residual sodium xylene sulfonate.
[0108] Process for producing EmImXS and for producing tetrabutylammonium xylenesulfonate (TBABXS) from tetrabutylammonium bromide and sodium xylenesulfonate, tetrabutylammonium benzoate from tetrabutylammonium bromide and sodium benzoate (TBABBz) in the same way.
[0109] The method includes the following steps. Add approximately equimolar amounts of 1-ethyl-3-methylimidazolium bromide or tetrabutylammonium bromide and sodium xylenesulfonate to a 2 L round bottom flask. Toluene (600ml, 5-6 times the mass of salt) and zeolite were added to the flask. The flask was fitted with a condenser and the slurry was heated at reflux overnigh...
Embodiment 2
[0111] Trihexyltetradecylphosphonium xylenesulfonate (P66614XS) was prepared using two different methods.
[0112] In the first method, an ion-exchange column was loaded with xylenesulfonate anion and a solution of trihexyltetradecylphosphonium chloride was passed several times until the eluent was free of chloride ions, which indicated complete progress. exchange. 1Hnmr (CDCl 3 )δ0.85-0.95 (m, 12H, CH 3 ), 1.20-1.35 (m, 48H, CH 2 ), 1.40-1.60 (m, 14H, P-CH 2 &Ar-CH 3 ), 6.90-7.30 & 7.60-7.90 (m, 3H, CH).
[0113] Electrospray mass spec: ES+483[P66614+] m / z. ES-185[XS-] m / z.
[0114] According to the second method, trihexyltetradecylphosphonium chloride (P66614 chloride; 1.1 mol) was refluxed overnight with 1 mol sodium xylenesulfonate (NaXS) in toluene. The product was filtered and the toluene was removed by rotary evaporation under vacuum to recover the ionic liquid. The ionic liquids were characterized by NMR / ES MS.
[0115] A hydrophobic ionic liquid based on thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com