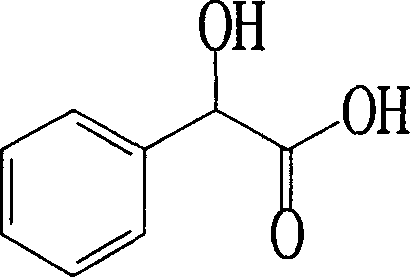
Process for preparing (R)-mandelic acid by microbial asymmetric resolution
A mandelic acid, asymmetric technology, applied in the field of biological separation of racemic compounds, can solve problems such as difficult separation, difficult separation of product and substrate, and many by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Cultivation of bacterial strains: The composition of the medium is that the components contained in each 100ml of culture solution are in g: glucose 0.25-1.0, meat extract 0.5-2.0, peptone 0.5-2.0, yeast extract 0.1-0.5, (Nh 4 ) 2 HPO 4 0.5~1.0, KH 2 PO 4 0.25~0.5, MgSO 4 ·7H 2 O 0.025~0.1, NaCl 0.001~0.005, ZnSO 4 ·7H 2 O0.001~0.005, FeSO 4 ·7H 2 O 0.001~0.005, CuSO 4 ·5H 2 O 0.0001~0.0005, MnSO 4 4H 2 O 0.0001~0.0005.
[0062] The culture conditions are as follows: the initial pH is 6.0-8.0, the filling volume is 20%, the culture temperature is 30° C., the rotation speed of the shaking flask is 100-300 rpm, and the culture time is 48 hours.
Embodiment 2
[0064] Preparation of whole cells: B.flavum AS 1.818 was inoculated in a 250ml shake flask with a liquid volume of 20%, and cultured at 30°C and 100-300rpm for 48 hours with shaking; after the cultivation, the cells in the fermentation broth were centrifuged and washed with normal saline Twice, the collected cells were stored in a 4°C refrigerator for splitting reactions.
Embodiment 3
[0066] Preparation of (R)-mandelic acid by asymmetric resolution by microbial method: in 2 mL of potassium phosphate buffer containing 10 mg of racemic mandelic acid at 0.2 mol / L, pH 7.0, add 0.1 g (i.e. weight to volume ratio 5%) B. flavumAS 1.818 bacterial cells, 25°C, 150 rpm, transformed for 72 hours, centrifuged the transformed solution, collected the supernatant, adjusted the pH to 1.0 with 3mol / L hydrochloric acid, extracted with 2.5 times the volume of ethyl acetate, and separated The ethyl acetate layer was dehydrated with anhydrous sodium sulfate, dried in vacuum at low temperature, and the residual solid was dissolved with a mixed solution of n-hexane and isopropanol (volume ratio of 9:1), and the product (R)-mandelic acid had an optical purity of 90.83 %e.e, yield 38.63%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com