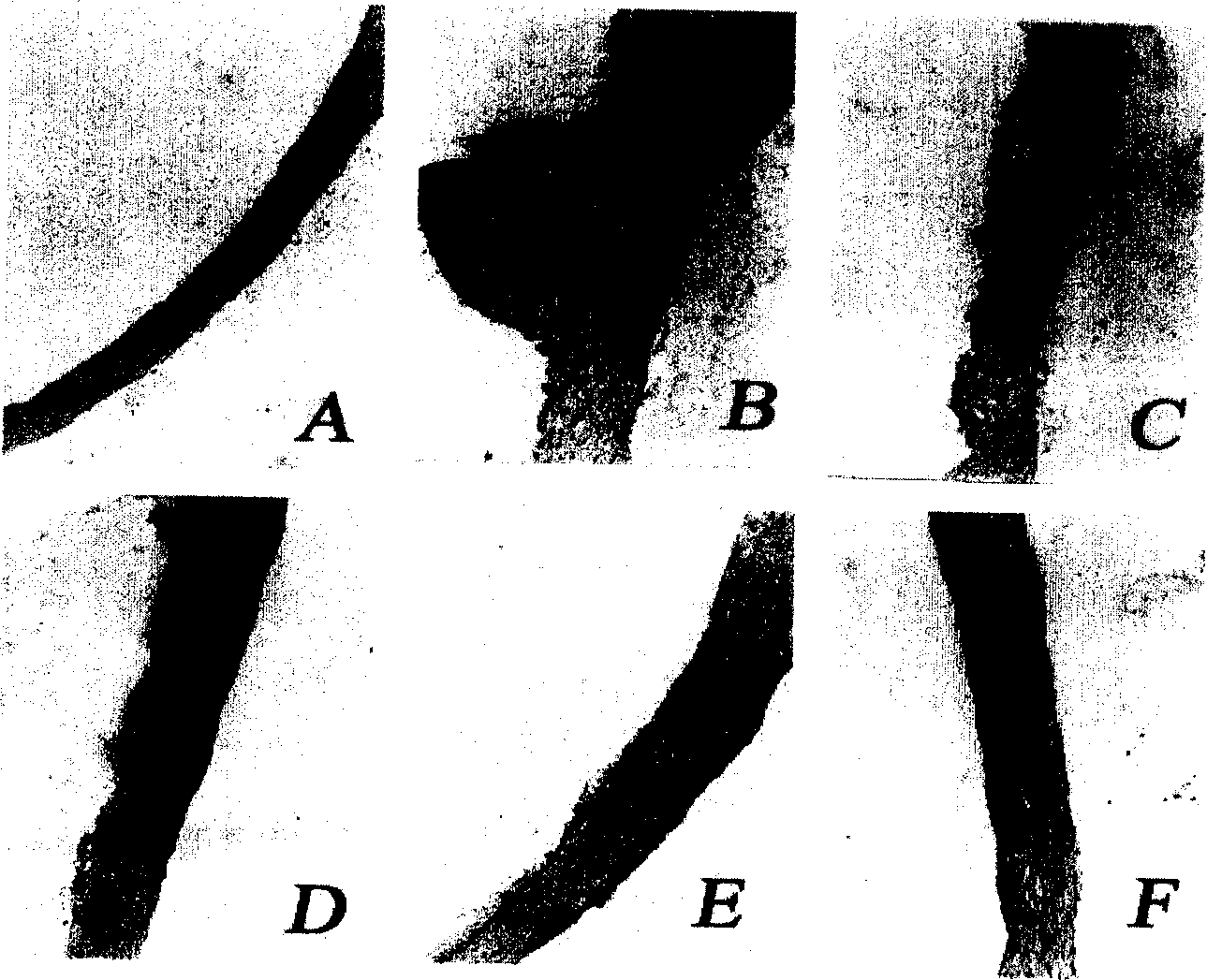Recombinant plasmid containing PON gene and its use
A technology of recombining plasmids and genes, applied in the field of biomedicine, can solve the problems of long course of disease and high incidence of diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Construction of recombinant plasmid vector
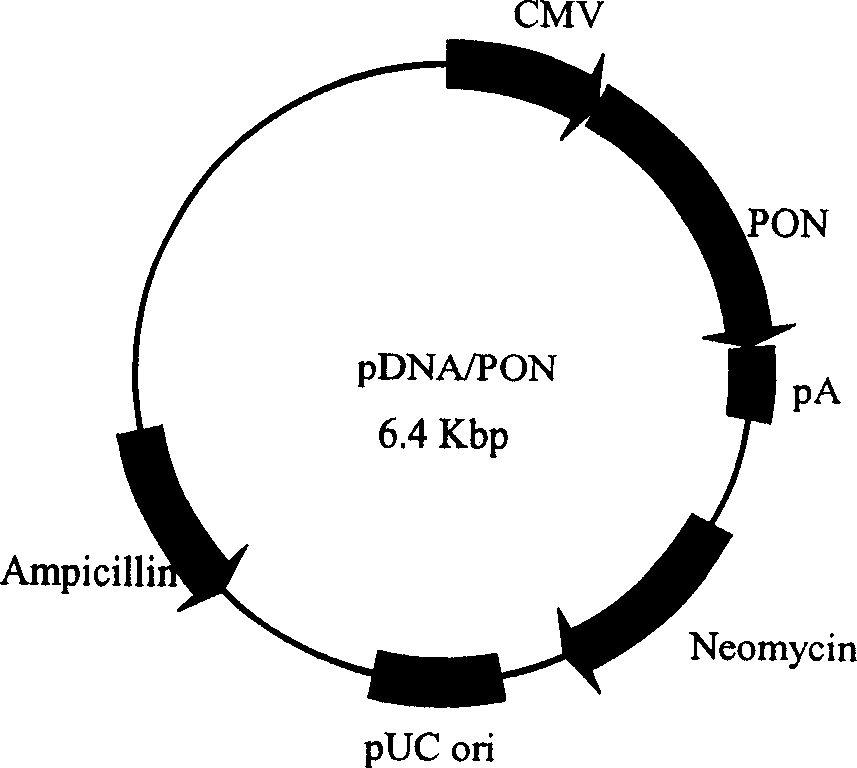
[0035] The prokaryotic expression vector pBluescrip / PON (gifted by Dr. Clement E. Furlong) containing the human PON gene (see SEQ ID NO: 1 sequence) was used as a template to design primers: primer 1, 5'GCA GGTACC ATGGCGAAGCTGATTGCG 3′; Primer 2, GCA GAATTC TTAGAGCTCACAGTAAAG 3'. KpnI and EcoRI restriction sites were inserted into primer 1 and primer 2, respectively. PCR amplification was carried out under 40 cycles of 94 C for 40 seconds, 58 C for 40 seconds, and 72 C for 2.5 minutes. The amplified product was digested with KpnI and EcoRI (under the action of T4 ligase) and inserted into pDNA, and sequenced after PCR and enzyme digestion identification were correct to obtain the pDNA / PON plasmid ( figure 1 ). The plasmid was amplified in Escherichia coli TOP10 strain, and the plasmid was extracted.
[0036] In addition, according to the PON gene sequence disclosed herein or other sequences, those skilled in the art ca...
Embodiment 2
[0038] Prevention of soman poisoning by pDNA / PON
[0039] The organophosphorus compound soman is a highly toxic military agent and can also be used in terrorist activities. Its toxicity is mainly manifested as the irreversible inhibition of cholinesterase, which phosphorylates the serine residue in the enzyme active site of cholinesterase, leading to the inactivation of the enzyme and the accumulation of acetylcholine in the nerve endings. PON can catalyze the hydrolysis of the phosphoester bond in the soman molecule and protect against a certain degree of soman poisoning (Table 1). In this experiment, the plasmid pcDNA / PON was directly injected intravenously into the mice, and 200 μg / kg (1.0 LD) of sc Soman was given to the mice 24 hours later. The normal control and mice injected with empty plasmids showed muscle tremors, vertical tail, tremor, and salivation. , convulsions, and died within about 9 minutes, while 60% of mice pre-injected with 600 μg pcDNA / PON survived (obse...
Embodiment 3
[0041] pDNA / PON prevents atherosclerosis
[0042] The surface of normal vascular intima is smooth without lipid infiltration. After feeding with high-fat diet for 25 days, the aortic intima had serious pathological changes, the surface was rough, and lipid plaques were formed higher than the surface of the intima, especially in the aortic root, where obvious lipid plaques appeared. It shows that pathological changes of atherosclerosis have been formed. If given (tail vein injection) model rats pcDNA / PON for 125 days at the same time, the plaques on the wall of the aorta decreased or disappeared, and the pathological changes were greatly improved ( figure 2 ). It shows that the recombinant plasmid genome can be effectively expressed in mice, and the PON in the expression product has played an antioxidant role in the serum, increasing the level of SOD, reducing the production of lipid peroxidation products, and reducing serum TG, LDL-C And TC, remove fat accumulation in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com