Kidney target precursor medicine, said prepn., its preparing method and application
A prodrug and drug technology, applied in the field of pharmacy, can solve the problems of digestive system damage, reducing inflammatory cells, pathogenic bacteria not easy to localize and phagocytosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Accurately weigh 100 mg of triptolide, 200 mg of succinic anhydride, and 10 mg of dimethylaminopyridine, add 2 ml of anhydrous pyridine, and 1 ml of triethylamine, react overnight at room temperature, and monitor the reaction on a silica gel plate. After the reaction was completed, 50 ml of pure water was added, and the pH was adjusted to acidity with hydrochloric acid. 20ml of chloroform was extracted 3 times, the extracts were combined, concentrated in vacuo at room temperature, purified by silica gel column chromatography twice, the product was collected, concentrated in vacuo and dried to obtain 117.6 mg of off-white solid triptolide succinate with a yield of 91.5%. The structure of the product was tested by UV, MS, FTIR, 1 Confirmed by H-NMR. mp 109~111℃; IR(KBr)3463(-COOH)cm -1 ; MS m / z 459 (M-H) + ; 1 H-NMR (400MHz, CDCl 3 ): 5.06 (1H, s, -14CH), 4.67 (2H, s, 19-CH 2 ), 3.82 (1H, d, 11-CH), 3.50 (1H, d, 12-CH), 3.43 (1H, d, 7-CH), 2.75 (5H, m, CH 2 CH 2 ,...
Embodiment 2
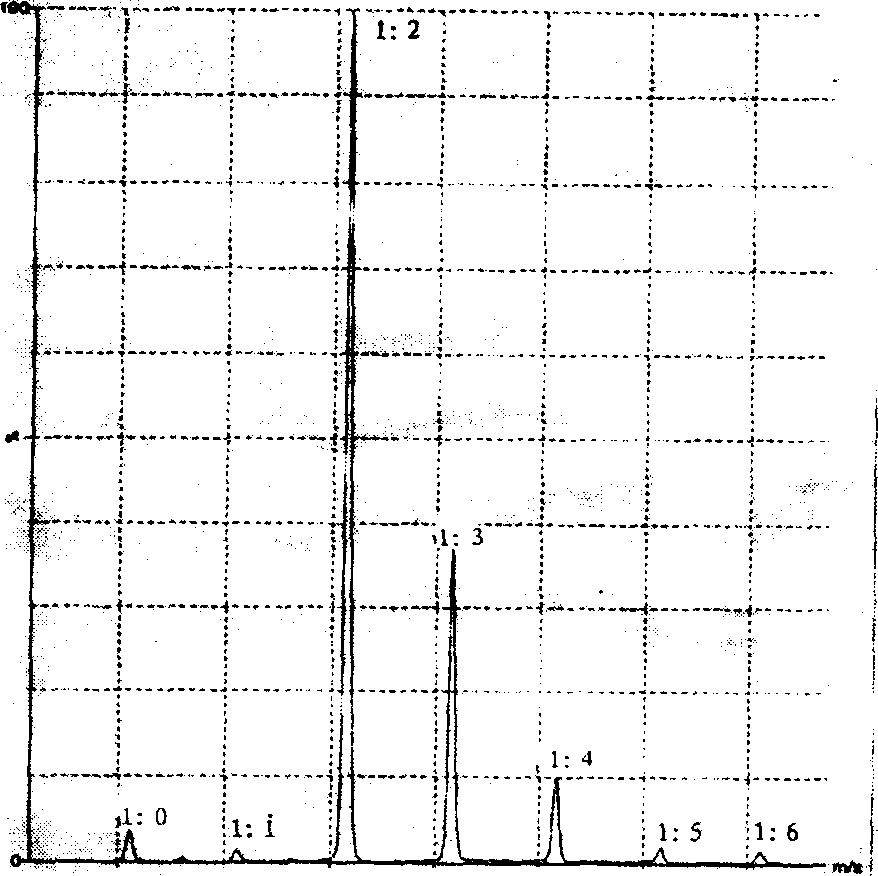
[0052] Accurately weigh 100 mg of lysozyme, dissolve in 5 mL of 0.1 mol / l borate buffer, dissolve 100 mg of EDC, 50 mg of HOBT and 100 mg of triptolide succinate in 0.5 ml of acetonitrile. Slowly add the acetonitrile solution dropwise into the borate buffer solution of lysozyme, stir at a moderate speed, and react at 0°C for 24 hours. The reaction solution was centrifuged for 10 minutes (2,000 rpm, 0°C) to remove insoluble matter, and unreacted triptolide succinate and small molecular impurities were removed by Sephadex G-25. The protein-containing effluent was collected and freeze-dried to obtain a white loose powder, which was sealed and stored at -20°C. The binding rate and prodrug concentration of lysozyme to triptolide succinate were determined by MS and HPLC, and the mass spectrum is shown in the attached figure 1 , found that the binding ratio was 1:1 to 1:7, and the HPLC hydrolysis assay showed that the binding ratio was 1:2.3, which was consistent with the mass spec...
Embodiment 3
[0054] Accurately weigh 100mg of insulin, dissolve in 5mL of 0.1mol / L borate buffer, dissolve 100mg of EDC, 50mg of HOBT and 100mg of triptolide succinate in 0.5ml of acetonitrile. Slowly add the acetonitrile solution dropwise into the borate buffer solution of insulin, stir at a moderate speed, and react at 0°C for 24 hours. The reaction solution was centrifuged for 10 minutes (2,000 rpm, 0°C) to remove insoluble matter, and unreacted triptolide succinate and small molecular impurities were removed by Sephadex G-25. The protein-containing effluent was collected, the binding rate of insulin to triptolide succinate and the prodrug concentration were determined, and freeze-dried to obtain a white loose powder, which was sealed and stored at -20°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com