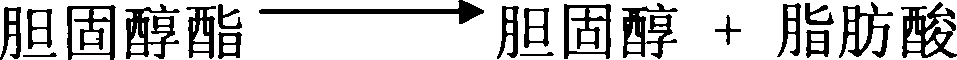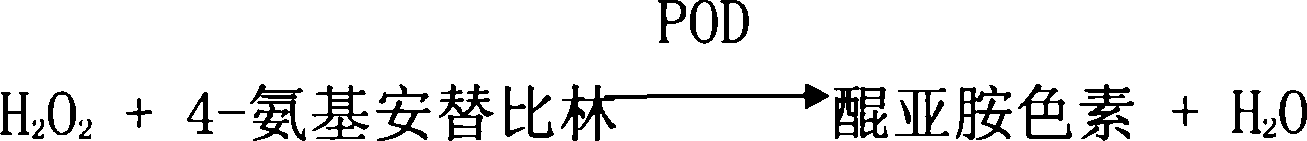Method for determining cholesterol concentration in remnant lipoprotein by immunobinding direct process or immuno-precipitation separation process
A lipoprotein and cholesterol technology, applied in the field of serum determination, can solve the problems of cumbersome, difficult and time-consuming operation in ordinary laboratories
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Detection of RLP by Immunobinding Direct Method
[0045] Use reagent I and reagent II, reagent I contains: 50mmol / L Tris-HDL buffer, 60U / L cholesterol lipase, 60U / L cholesterol oxidase, 150mmol / L peroxidase, 2g / L polyethylene glycol 6000 , 11.3mL rabbit anti-human ApoB100 antiserum, 7.5mL rabbit anti-human ApoA I antiserum, reagent II contains 150mmol / L peroxidase, 0.06mmol / L 4-aminoantipyrine and 50mmol / L Tris-HDL buffer liquid. The components of reagents I and II were added to the buffer solution according to their component contents, mixed evenly, and the precipitate was filtered off, and the obtained solution was stored at 4° C. for future use.
[0046] Take out 24uL serum or plasma from the test individual, add 225uL reagent I to the serum or plasma, mix well, and place at 37°C for 5 minutes. Allow ApoB100, ApoA I antigens to fully combine with antibodies. On the 722-type spectrophotometer, use a 505nm wavelength and a 1cm optical diameter cuvett...
Embodiment 2
[0050] Example 2 Detection of RLP by Immunoprecipitation
[0051] Use 113uL rabbit anti-human ApoB100 antiserum, 71uL rabbit anti-human ApoA I antiserum and 30uL serum or plasma to mix at room temperature or 37°C for 30 minutes, centrifuge at 4000 rpm to take the supernatant, and the obtained supernatant Save for later.
[0052] Take out 24uL supernatant, add 225uL reagent I to the supernatant, mix well, and place at 37°C for 5 minutes. On the 722-type spectrophotometer, use a 505nm wavelength and a 1cm optical diameter cuvette to zero with distilled water, and measure its absorbance value A 1 .
[0053] Then, add 75uL of reagent II to it, and keep it warm at 37°C for 5 minutes. On a 722-type spectrophotometer, use a 505nm wavelength and a 1cm optical diameter cuvette to zero with distilled water, and measure its absorbance value A 2 .
[0054] Use the known concentration c.f.a.s. cholesterol calibrator as the calibration solution, take 24uL and follow the same...
Embodiment 3
[0057] Linearity, reaction process, precision, recovery rate and interference test of the detection method of the present invention
[0058] 1. Linear Analysis
[0059] Take a low-value sample (RLP-C is 0.08mmol / L) and a high-value sample (RLP-C is 2.00mmol / L) and mix them equally to prepare the RLP-C of the median sample to be 1.04mmol / L. The RLP-C of two horizontal specimens was prepared by mixing equal volumes of samples with high value and low value respectively, which were 1.52mmol / L and 0.56mmol / L respectively. Samples of 5 different levels were measured 4 times with the method of Example 1 in descending order by two clearance methods respectively, and the linear analysis was carried out according to the NCCLS EP6-P file. Results The linear regression analysis equation was: Y=0.986X+0.019, r=0.992, indicating that the method had a good linear relationship within 2.00mmol / L.
[0060] 2. Intra-batch accuracy
[0061] Take samples of 3 different levels of RLP-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com