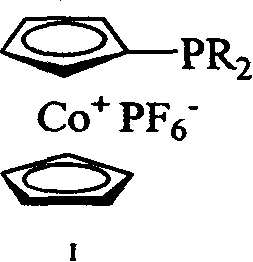
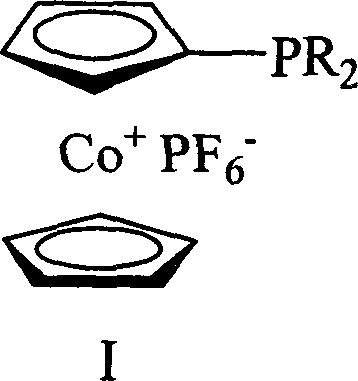
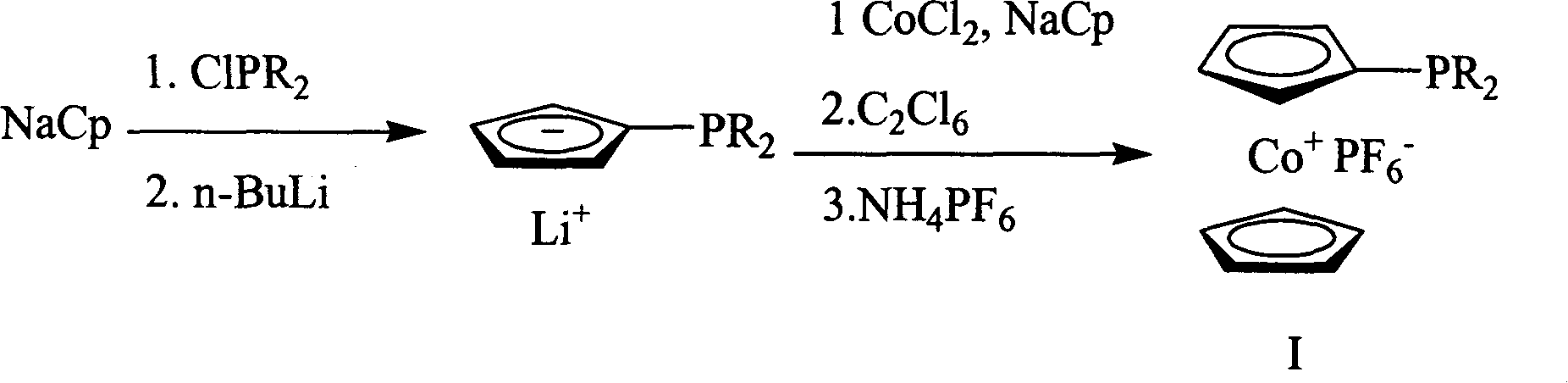Cobaltocene cation monophosphine ligand and its synthesis and uses
A technology of cobalt cations and ions, which can be used in organic compound/hydride/coordination complex catalysts, metallocenes, organic chemistry, etc., and can solve the problem of rare phosphine ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of dicyclohexylphosphinocene cobalt hexafluorophosphate (expressed as I-1)
[0020] At -30°C, 0.0105 moles of dicyclohexylphosphine chloride (ClPCy 2 ), after stirring at room temperature for 1.5 hours, 0.0105 moles of n-butyl lithium was added dropwise to the reaction system at -78°C, returned to room temperature, and continued to stir for 1.5 hours. Add 0.0105 moles of cobalt chloride, the solution turns dark brown, and reflux overnight. Cool to room temperature, add 0.013 moles of hexachloroethane, the solution turns green, react for 10 minutes, remove the solvent under reduced pressure, dissolve the residue with 50 milliliters of dichloromethane, pass through a diatomaceous earth chromatographic column, and filter off lithium chloride. Obtain a green oil; dissolve the oil with dichloromethane, add 0.0105 moles of ammonium hexafluorophosphate aqueous solution, stir for 1 hour, no precipitation occurs; add an equal volume of water to the solution, then ex...
Embodiment 2
[0025] Preparation of diisopropylphosphinocene cobalt hexafluorophosphate (expressed as I-2)
[0026] At -30°C, add 0.0105 moles of diisopropylphosphine chloride (ClP(i-Pr) 2 ), after stirring at room temperature for 1.5 hours, 0.0105 moles of n-butyl lithium was added dropwise to the reaction system at -78°C, returned to room temperature, and continued to stir for 1.5 hours. Add 0.0105 moles of cobalt chloride, the solution turns dark brown, and reflux overnight. Cool to room temperature, add 0.013 moles of hexachloroethane, the solution turns green, react for 10 minutes, remove the solvent under reduced pressure, dissolve the residue with 50 milliliters of dichloromethane, pass through a diatomaceous earth chromatographic column, and filter off lithium chloride. Obtain a green oil; dissolve the oil with dichloromethane, add 0.0105 moles of ammonium hexafluorophosphate aqueous solution, stir for 1 hour, no precipitation occurs; add an equal volume of water to the solution, t...
Embodiment 3
[0031] Preparation of di-tert-butylphosphinocene cobalt hexafluorophosphate (expressed as I-3)
[0032] At -30°C, add 0.0105 moles of di-tert-butylphosphine chloride (ClP(t-Bu) 2 ), after stirring at room temperature for 1.5 hours, 0.0105 moles of n-butyl lithium was added dropwise to the reaction system at -78°C, returned to room temperature, and continued to stir for 1.5 hours. Add 0.0105 moles of cobalt chloride, the solution turns dark brown, and reflux overnight. Cool to room temperature, add 0.013 moles of hexachloroethane, the solution turns green, react for 10 minutes, remove the solvent under reduced pressure, dissolve the residue with 50 milliliters of dichloromethane, pass through a diatomaceous earth chromatographic column, and filter off lithium chloride. A green oil was obtained; the oil was dissolved in dichloromethane, and a 0.0105 mole aqueous solution of ammonium hexafluorophosphate was added, and stirred for 1 hour, but no precipitate was formed. Add an eq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com