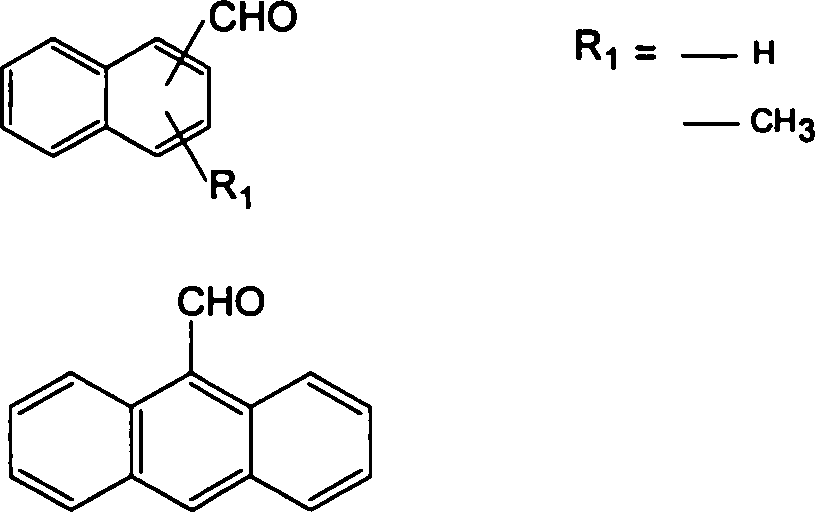
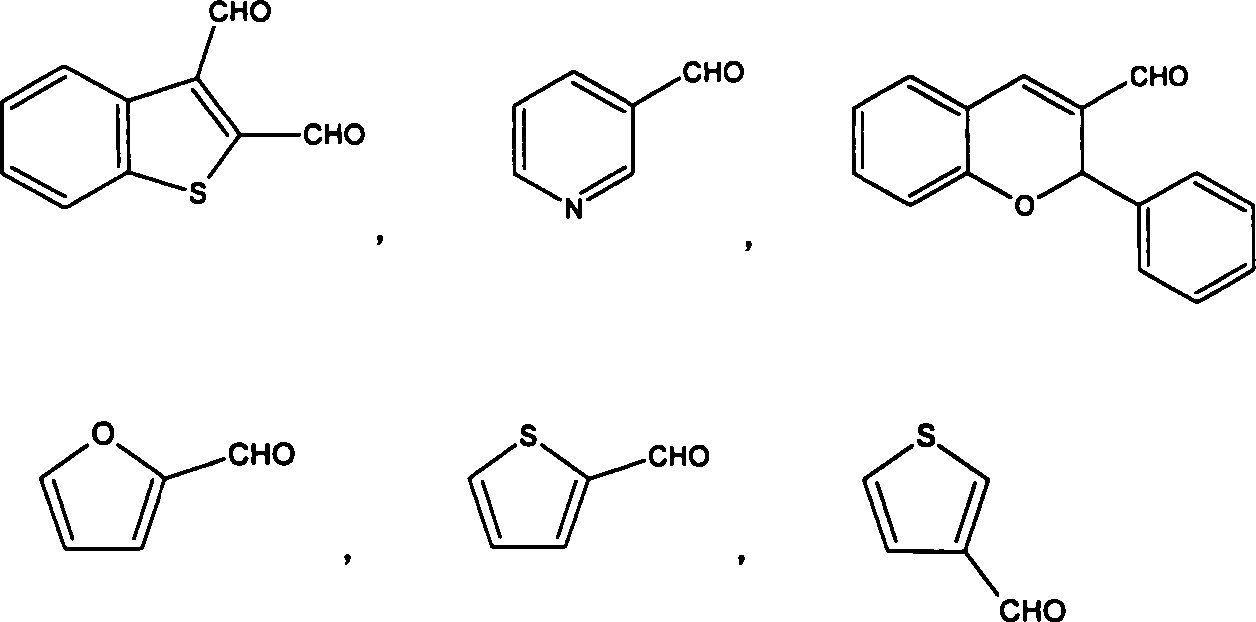
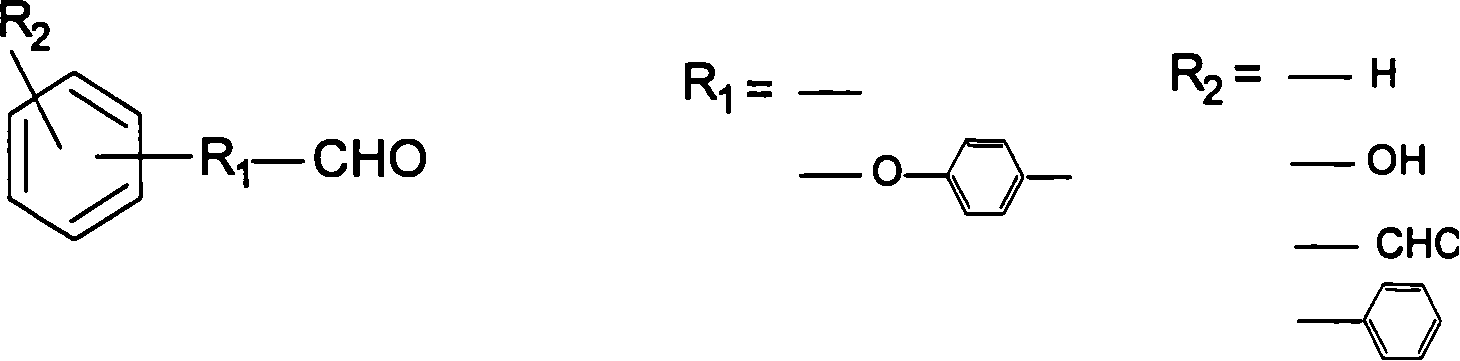Thermostable benzoxazine resin compound and its preparation method and uses
A technology of resin compound and benzoxazine, which is applied in the field of heat-resistant benzoxazine resin compound and its preparation, can solve the problems of expensive monomer, limited application, and insignificant improvement of heat resistance, etc., and achieves Effects of increasing cross-linking density, improving heat resistance, and increasing carbon residue rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 prepares terephthalaldehyde (TPA) / diamine type benzoxazine (B-BOZ) compound
[0044] Stir and mix 90g of diamine-type benzoxazine (B-BOZ) and 10g of terephthalaldehyde (TPA) at a temperature of about 90°C, and then vacuumize at a temperature of 130°C for 0.5 hours at a vacuum degree of 0.05-0.095MPa. 2 hours at 140°C, 3 hours at 150°C, 1 hour at 160°C, 1 hour at 170°C, 1 hour at 180°C, 1 hour at 190°C, 1 hour at 200°C, 1 hour at 220°C ℃ for 1 hour to obtain a cured product with a glass transition temperature of 240 °C, a tensile strength of 75 MPa, a tensile modulus of 4.7 GPa, an elongation at break of 1.8%, a flexural strength of 115 MPa, and a flexural modulus of 4.7 GPa . The mass ablation rate is 0.0201g / s. The cured product is thermally analyzed by TGA, and the test results are shown in Table 1
Embodiment 2
[0045] Example 2 Preparation of aldehyde-containing monocyclic benzoxazine (AS-BOZ) / diamine-type benzoxazine (B-BOZ) complex
[0046] Diphenylmethane diamine type benzoxazine (B-BOZ) 77g and 3-phenyl-6-formyl-3,4-dihydro-2H-1,3-benzoxazine (AS-BOZ) Mix 23g at a temperature of 90°C, then vacuumize at a temperature of 130°C for 0.5 hours at a vacuum degree of 0.05-0.095MPa, at 140°C for 1 hour, at 150°C for 2 hours, at 160°C for 1 hour, at 170°C 1 hour at 180°C, 1 hour at 190°C, 1 hour at 200°C, 1 hour at 220°C after curing. After TGA thermal analysis, the test results are shown in Table 1
Embodiment 3
[0047] Embodiment 3 prepares furfural and diamine type benzoxazine (B-BOZ) compound
[0048] Stir and mix 91g of diamine-type benzoxazine (B-BOZ) and 9g of furfural (Furfural) at a temperature of about 90°C, and then vacuumize at a temperature of 130°C at a vacuum degree of 0.05-0.095MPa for 0.5 hours, and then at 130°C 3 hours at 140°C, 1 hour at 140°C, 1 hour at 150°C, 1 hour at 160°C, 1 hour at 170°C, 2 hours at 180°C, 1 hour at 200°C, 1 hour at 220°C Finally, after TGA thermal analysis, the test results are shown in Table 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com