Human amnion cell capable of expressing extraneous gene and its preparation method and uses
An exogenous, human amniotic membrane technology, applied in the field of human amniotic membrane cells, can solve the problems of difficult transgenic operation, large cell damage, low preparation titer, etc., and achieves broad clinical application prospects and development value, reduced ischemic area, and safe. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The cultivation of embodiment 1 human amnion cells
[0057] After the human amniotic membrane is stripped from the placenta of a healthy cesarean pregnant woman, scrape off the sponge layer and fibroblast layer below, cut the membrane into pieces with scissors, add 0.25% trypsin / EDTA, digest for half an hour, and then add serum to stop After centrifugation, the obtained cells were inoculated on a 6-well plate, and RPMI 1640 medium (10% fetal bovine serum, streptomycin 100 μg / ml, penicillin 100 U / ml and glutamine 0.3 mg / ml) was added at 37°C containing 5% CO 2 Cell culture incubator.
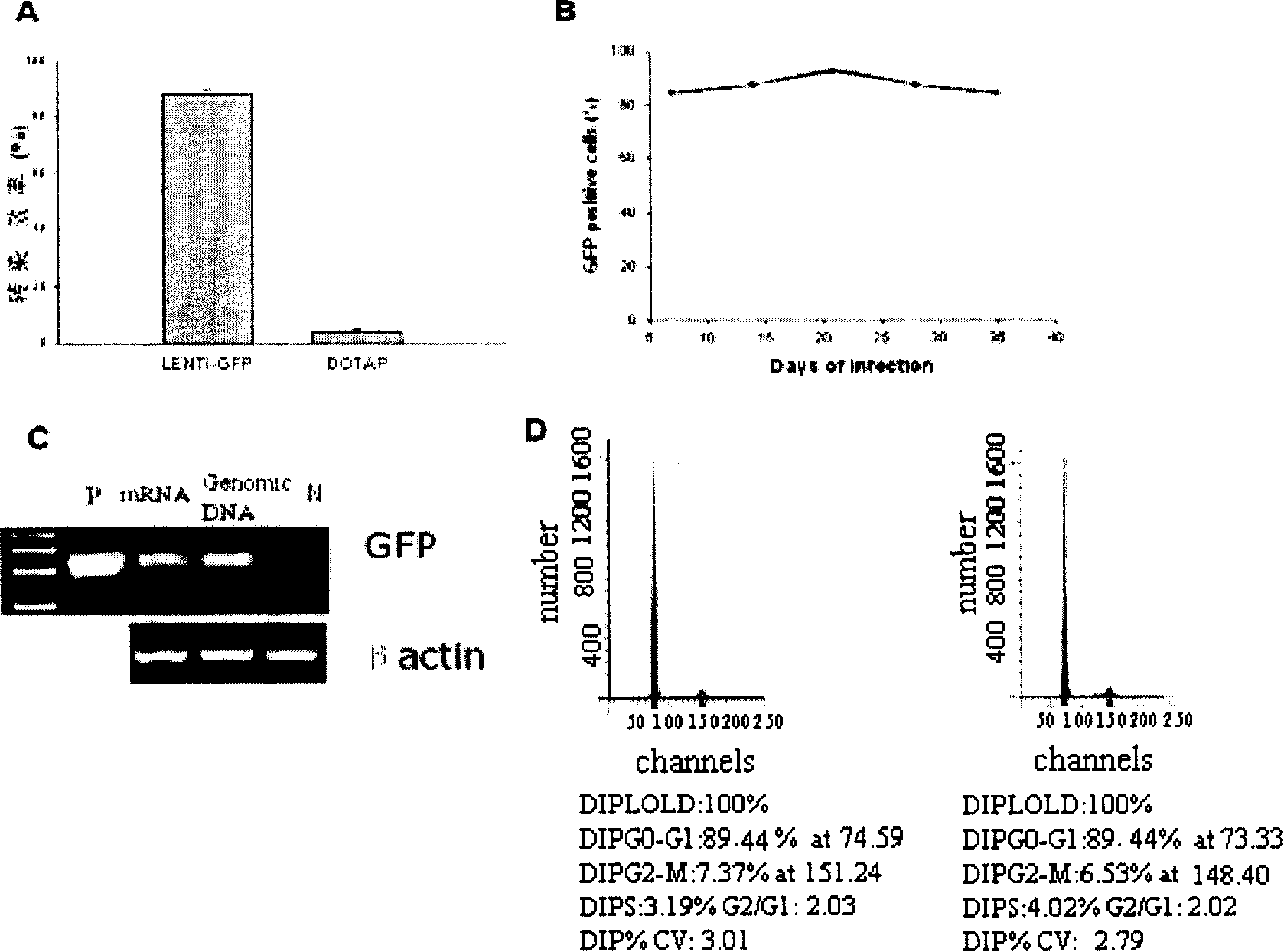
[0058] Example 2 Flow cytometry (FACS) and PCR analysis virus infection amnion cell efficiency
[0059] hAEC at 2×10 5 The density of cells / well was seeded in a 6-well plate. After 48 hours, lentivirus PWPT (MOI=100) was added, and 10 μg / ml polybrene was added at the same time. The transfection was treated for 24 hours, then washed with PBS for 3 times, and con...
Embodiment 3
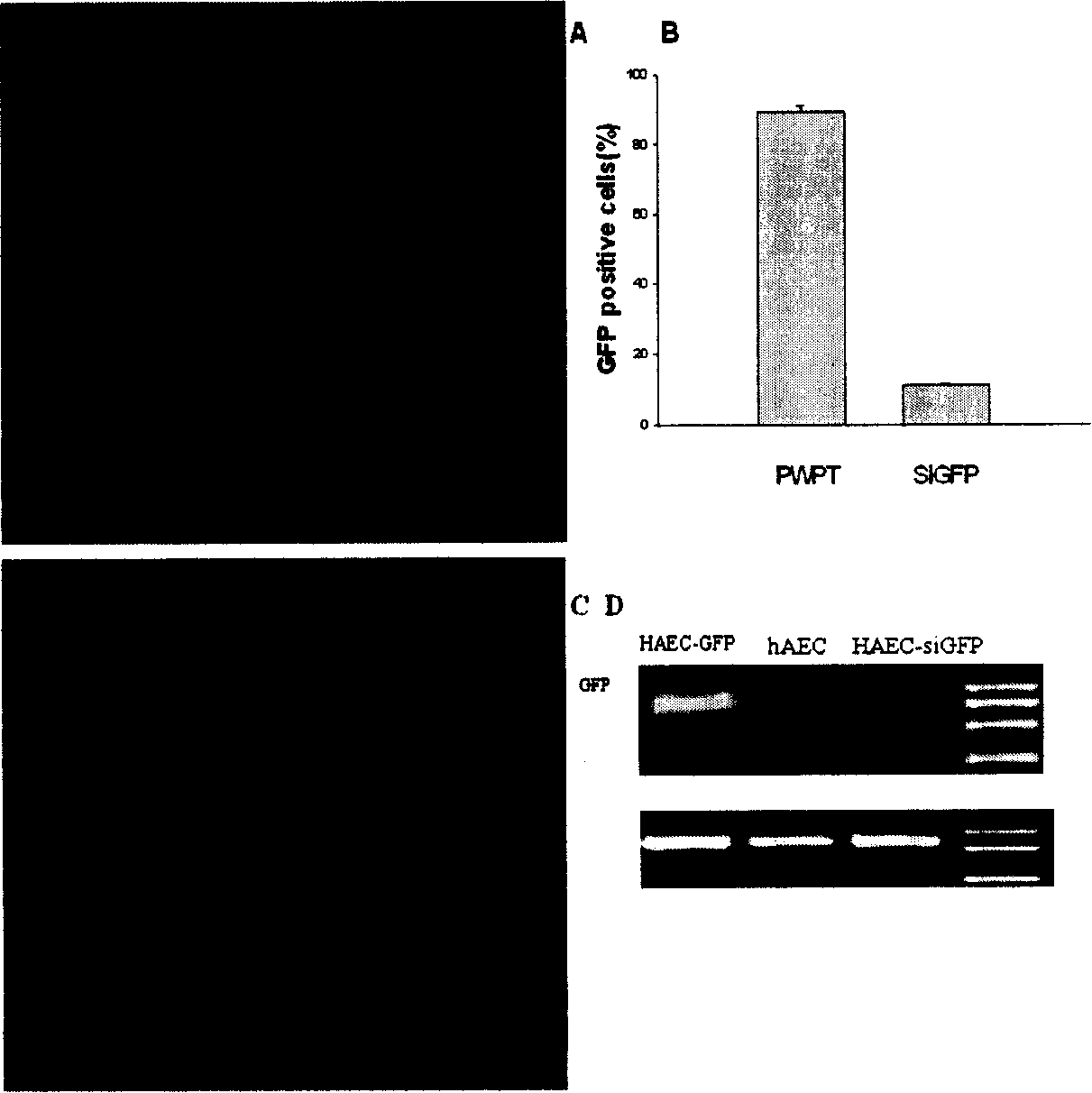
[0062] Example 3 Lentivirus-mediated RNAi inhibits the expression of EGFP in amnion cells
[0063] RNAi is considered to be the most effective method of gene silencing at the post-transcriptional level. Through the method of using lentivirus to transfer EGFP and siGFP into amnion cells, it was found that the expression of lentivirus-mediated RNAi can inhibit specific genes in cells at the mRNA level. The siGFP sequence is transcribed in the cell by the lentivirus PLVTHMsiGFP to form a double-stranded RNA, which mediates the excision of the EGFP mRNA in the cell.
[0064] hAEC at 2×10 5 The density of the cells / well was seeded in a 6-well plate. After 48 hours, the lentivirus PLVTHM (MOI=100) was added, and 8 μg / ml polybrene was added at the same time. After 24 hours of transfection, pLVTHMsiGFP was added, treated for 24 hours, and then washed 3 times with PBS , replace with new RPMI 1640 medium to continue culturing.
[0065] After one week of cell culture, the expre...
Embodiment 4
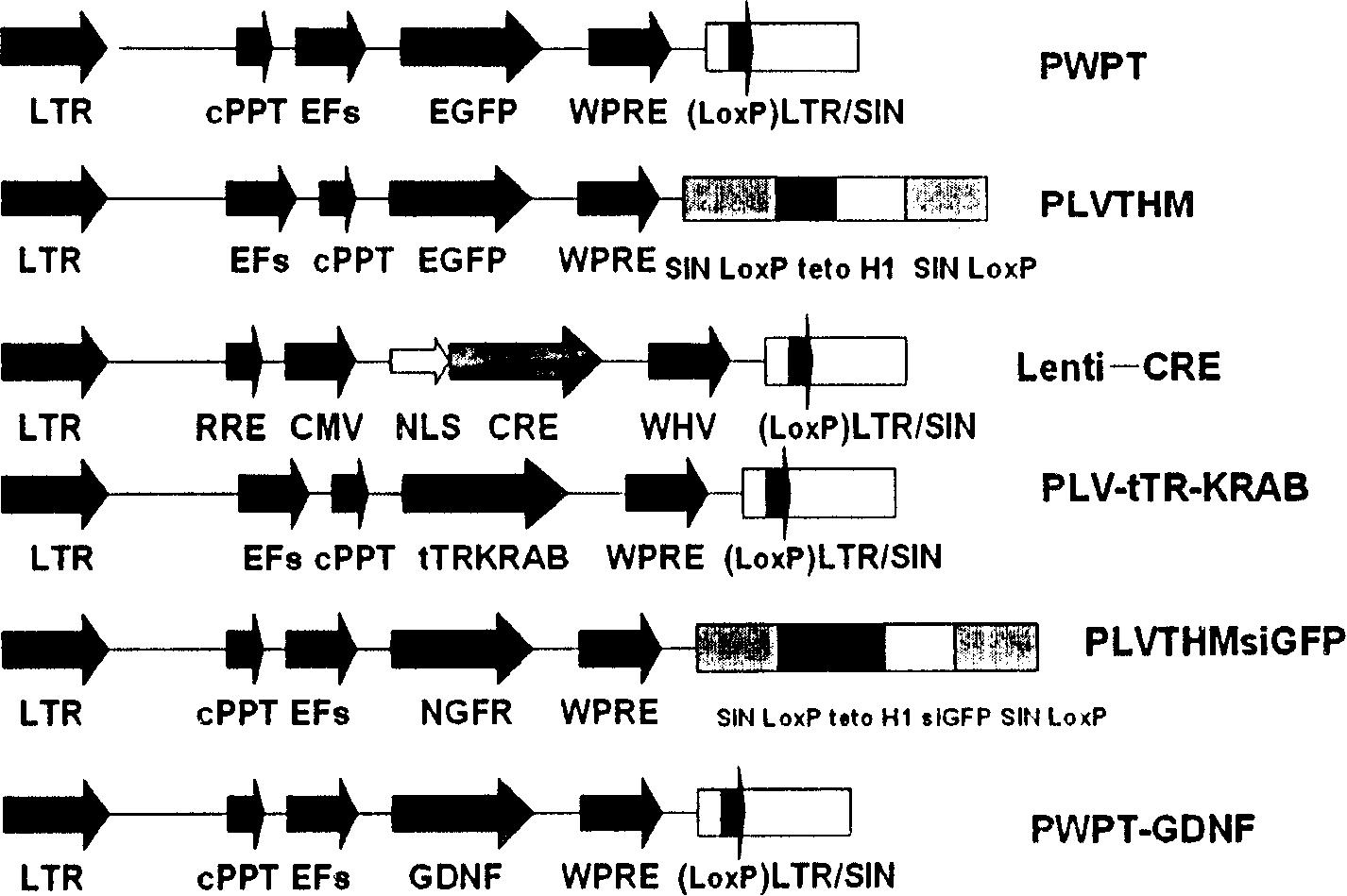
[0067] Example 4 The lentivirus-mediated CRE-LoxP system effectively extracts the transferred foreign gene
[0068] Cre is a site-specific recombinase in the integrase family, which can catalyze the recombination of fragments between loxP (Sternberg, N. (1981) J.Mol.Biol.150, 467-486), using lentivirus-mediated CRE -LoxP system specifically removes foreign genes in amnion cells. The 3' end of the lentivirus has a LoxP site, and after reverse transcription and integration into the genome, both ends have a LoxP site, and the CRE enzyme can remove the fragments between the two ends.
[0069] hAEC at 2×10 5 The density of the cells / well was seeded in a 6-well plate, and after 48 hours, lentivirus PWPT (MOI=200) was added, and 8 μg / ml polybrene was added at the same time, transfected for 24 hours, then Lenti-CRE was added, treated for 24 hours, and then washed with PBS for 3 hours The second time, replace with new RPMI 1640 medium to continue culturing.
[0070] After one week o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com