Novel alanyl-amino peptidase inhibitors for functionally influencing different cells and treating immunological, inflammatory, neuronal, and other diseases
A technology of compounds and general formulas, applied in the fields of extracellular fluid diseases, nervous system diseases, anti-inflammatory agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Inhibition Characteristics of New Inhibitors of Alanyl Aminopeptidase
[0065] In Tables 1 to 14 below, new inhibitors are summarized, for which the inventors have shown that these substances are capable of inhibiting alanyl aminopeptidase and enzymes with similar effects in enzymatic activity. Inhibition characteristics were measured as IC-50 values or ID50 values (the latter marked with "*") for the enzymes in question. Via fluorescent substrate / product (Ala) 2 -Rhodamine 110 to measure enzyme activity.
[0066] Table 1:
[0067]
[0068]
[0069] Table 2:
[0070]
[0071]
[0072] table 3:
[0073]
[0074]
[0075]
[0076]
[0077]
[0078]
[0079]
[0080]
[0081]
[0082]
[0083]
[0084]
[0085]
[0086]
[0087]
[0088]
[0089]
[0090]
[0091]
[0092]
[0093]
[0094]
[0095]
[0096]
[0097]
[0098]
[0099]
[0100]
[0101] ...
Embodiment 2
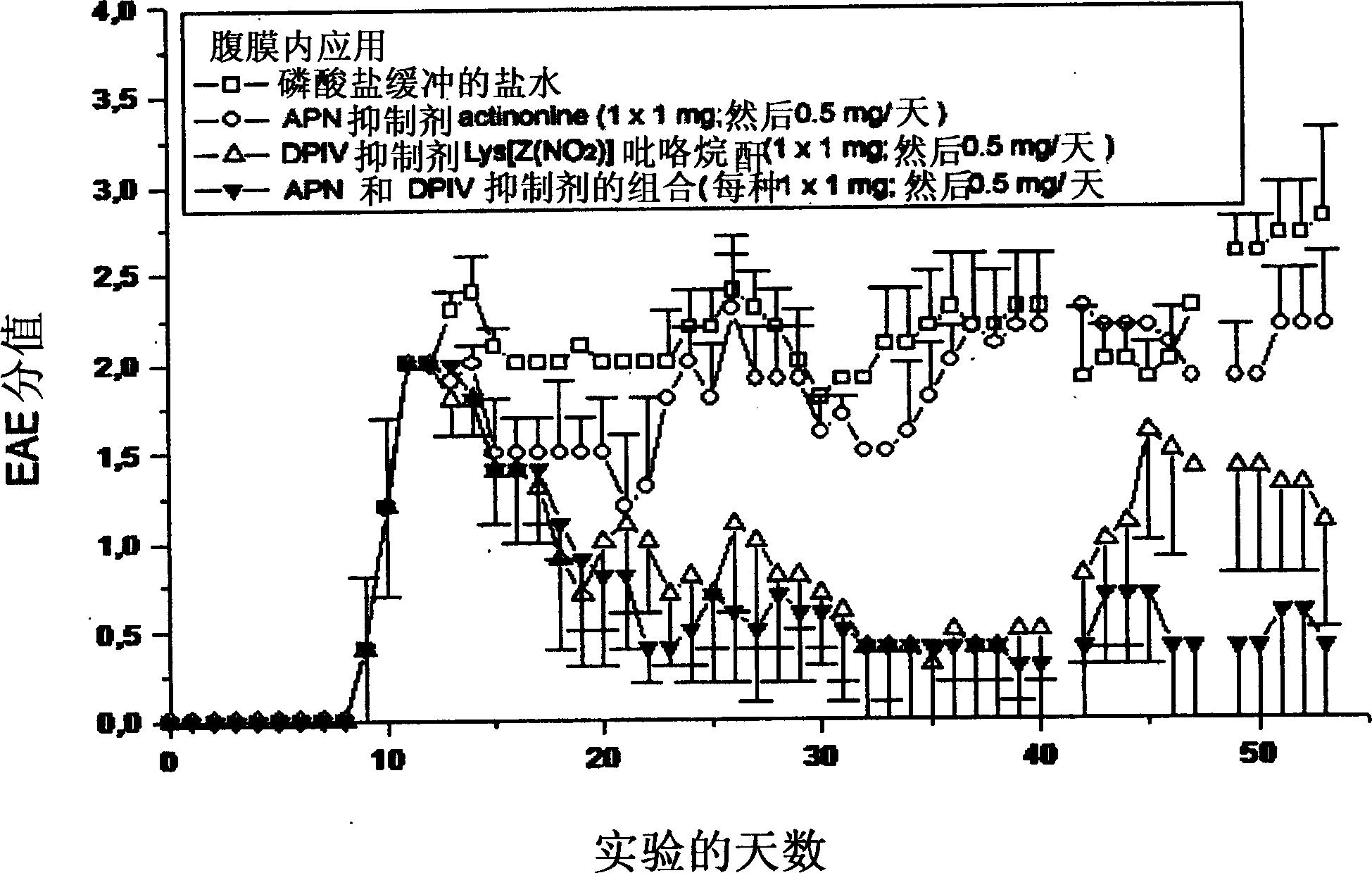
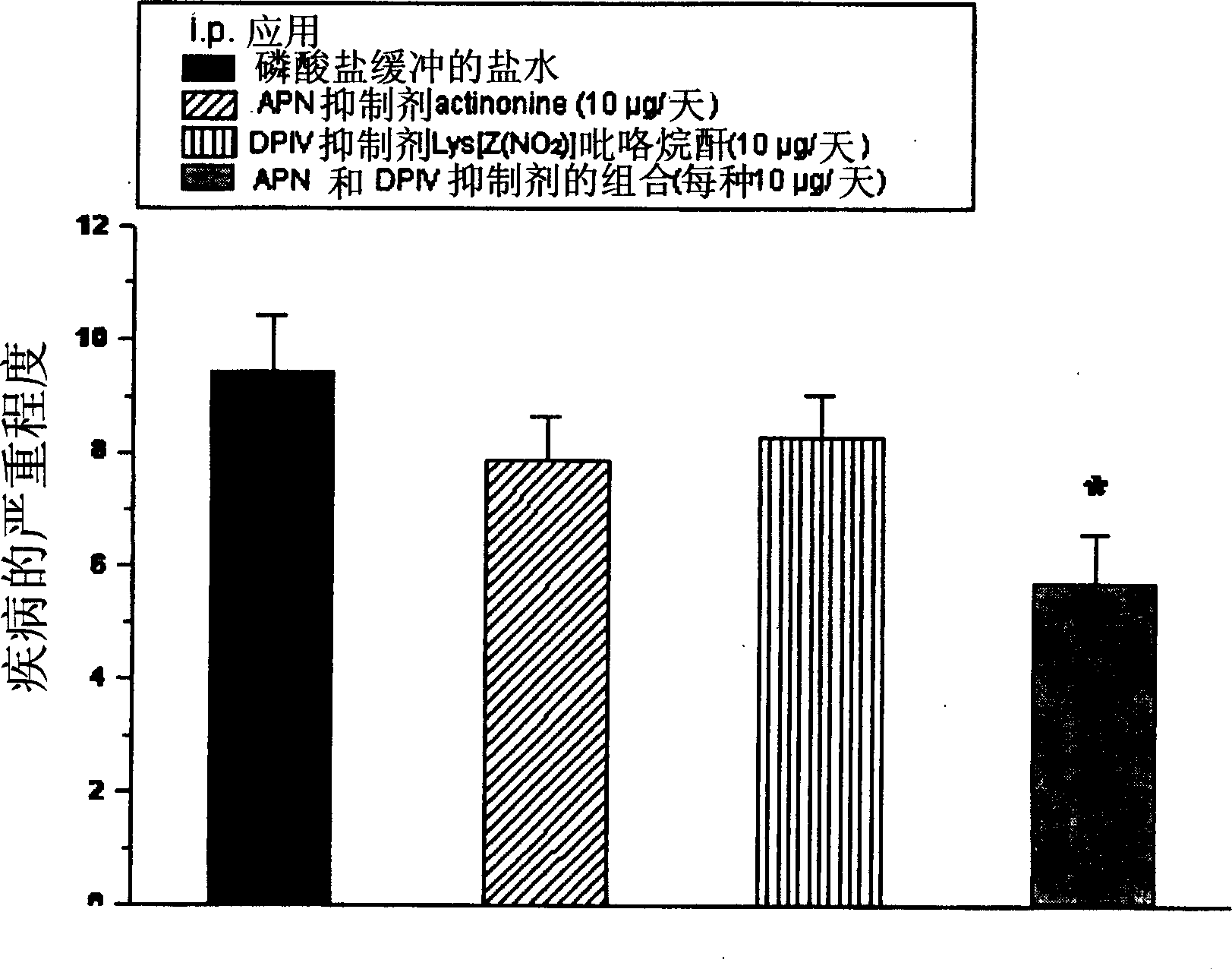
[0218] Combined inhibition of alanyl aminopeptidase and similar enzymes and dipeptidyl peptidase IV and similar enzymes against experimental autoimmune encephalomyelitis (EAE) in mice, an animal model of multiple sclerosis treatment effect
[0219] The disease EAE was induced by daily injection of PLP139-151 (myelin antigen protein lipoprotein peptide 139-151) to SJL / J mice (n=10). Following disease onset, which is day 11 after immunization, therapeutic intervention was performed by intraperitoneal injection of 1 mg of each peptidase inhibitor on the first day and further 0.5 mg of each inhibitor every other day. Disease scores are defined by clearly varying degrees of paralysis [vD1]. Healthy animals have a disease score of 0. Actinonine is used as alanyl aminopeptidase inhibitor, Lys[Z(NO 2 )] pyrrolidine anhydride (pyrrolidide) is used as a dipeptidyl peptidase IV inhibitor. Treatment was performed 46 days after immunization. The results are shown in figure 1 middle. ...
Embodiment 3
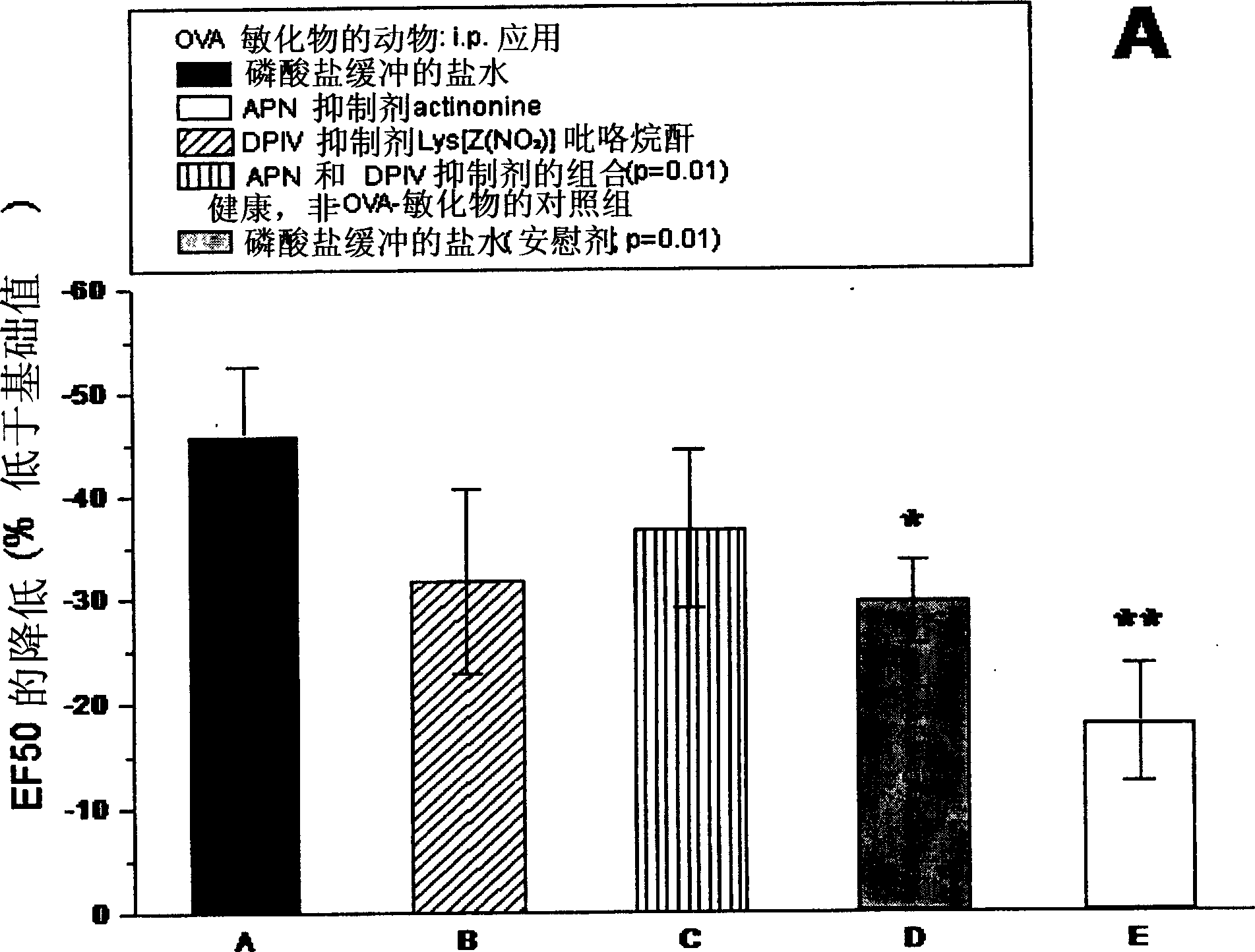
[0221] Combined inhibition of alanyl aminopeptidase and similar enzymes and dipeptidyl peptidase IV and similar enzymes against dextran sulfate-induced colitis (an animal model of chronic inflammatory small bowel disease) in mice treatment effect
[0222] Primary colon-associated inflammation (a disease equivalent to human ulcerative colitis) was induced in 8-week-old female Balb / c mice by administration of 3% dextran sodium sulfate dissolved in drinking water. After three days, all animals showed obvious symptoms typical of the disease. Peptidase inhibitors (or phosphate-buffered saline as placebo) were administered intraperitoneally from day 5 for three consecutive days. The extent of disease is determined according to a known rating system (score). When determining the score, the following parameters were considered: consistency of the stool (solid = 0 points (pts.); mushy = 2 pts.; liquid / as diarrhea = 4 pts.); detection of blood in the stool (no blood = 0 pts. ; occult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com