Antibiotic peptide Babu plaster and its preparing method
A technology of antimicrobial peptide and non-woven fabric, which is applied in the field of antimicrobial peptide cataplasm and its preparation, which can solve the problems of adhesive tape allergic reaction, toxicity, skin irritation, etc., and achieve good air permeability, suitable skin adhesion, and not easy The effect of drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 antimicrobial peptide cataplasm
[0030] Formula and its ratio (about 30ml in total):
[0031] Phase I solution: 5.5g (18.3%) of gum arabic, 2.5g (8.3%) of polyvinylpyrrolidone, 12g (40%) of glycerin, 2mL of water;
[0032] Phase II solution: gelatin 3g (10%), water 8mL
[0033] Antimicrobial peptide: 300ug (10ppm)
[0034] GKV-1: Lys Gly Ala Arg Lys Gly Ala Lys Arg Gln Gly Gly Lys Lys Val Ala Arg Lys Ala LeuLys Arg Ala Gly Lys
[0035] The remainder is water.
[0036] Preparation:
[0037] 1. Prepare phase I solution: disperse gum arabic and polyvinylpyrrolidone in glycerin and water, and stir well to form a paste to obtain phase I solution;
[0038] 2. Preparation of phase II solution: After fully swelling the gelatin in water, heat and dissolve in a water bath at 60°C to obtain phase II solution;
[0039] 3. Prepare the antimicrobial peptide aqueous solution: add the antimicrobial peptide to water to dissolve, and stir to obtain ...
Embodiment 2
[0041] Embodiment 2 antimicrobial peptide cataplasm in vitro bactericidal test
[0042] 1. Preparation of test samples:
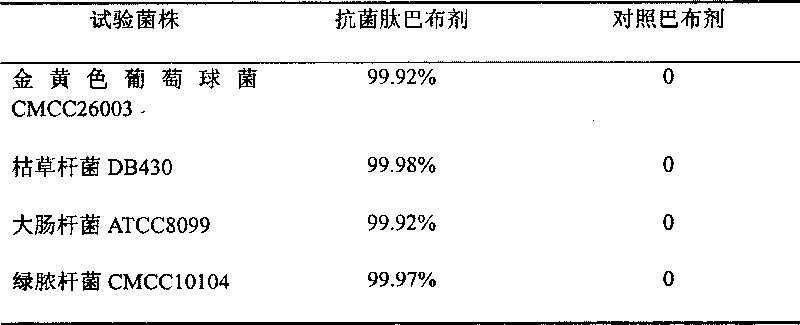
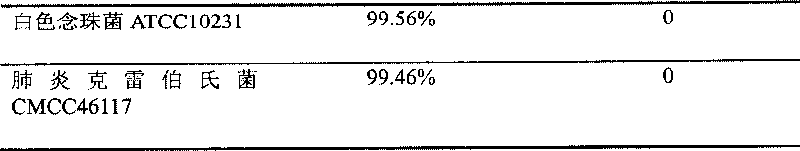
[0043] (1) antimicrobial peptide cataplasm sample: prepared by the method of Example 1,
[0044] Phase I solution: 5.5g gum arabic, 2.5g polyvinylpyrrolidone, 12g glycerin;
[0045] Phase II solution: 2.5-3g gelatin, 8mL water
[0046] Antimicrobial peptide: 300ug (10ppm) and the balance is water.
[0047] GKV-2: Lys Gly Gly Arg Lys Gly Ala Lys Arg Gln Gly Gly Lys Lys Lys Leu Ala Arg Lys AlaLeu Lys Arg Ala Gly Arg
[0048] (2) Control sample:
[0049] Phase I solution: 5.5g gum arabic, 2.5g polyvinylpyrrolidone, 12g glycerin;
[0050] Phase II solution: 2.5-3g gelatin, the balance is water.
[0051]2. Test strains: Staphylococcus aureus CMCC26003, Bacillus subtilis DB430, Escherichia coli ATCC8099, Klebsiella pneumoniae CMCC46117, Pseudomonas aeruginosa CMCC10104, Candida albicans ATCC10231. (The above strains are from China Institute for the Control...
Embodiment 3
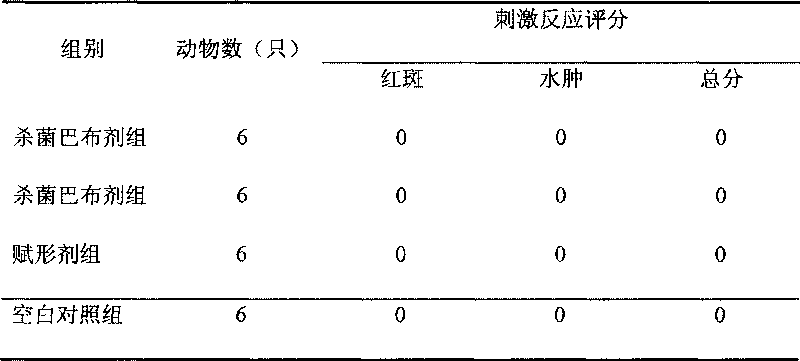
[0064] Example 3 Acute Toxicity Test in Animals
[0065] 1. Sample: antimicrobial peptide solution, that is, to dissolve the antimicrobial peptide in water so that the injection dose of the antimicrobial peptide reaches 1mg / kg. The sequences are:
[0066] GKV-2: Lys Gly Gly Arg Lys Gly Ala Lys Arg Gln Gly Gly Lys Lys Leu Ala Arg Lys Ala LeuLys Arg Ala Gly Arg
[0067] GKV-3: Lys Gly Gly Arg Lys Gly Ala Lys Arg Gln Gly Gly Lys Lys Leu Ala Arg Lys Ala LeuLys
[0068] 2. Experimental animals: 60 Kunming mice, half male and half male, weighing 33.5±0.25g, were provided by the Experimental Animal Department of Fudan University.
[0069] 3. Test method: intramuscularly inject mice once a day for 7 consecutive days, and observe the toxic reaction of the animals at the maximum dose. The experimental results showed that after 7 days of intramuscular injection, the animals had no abnormal reaction and their activities were normal. After 7 days of observation, all 60 mice survived. P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com