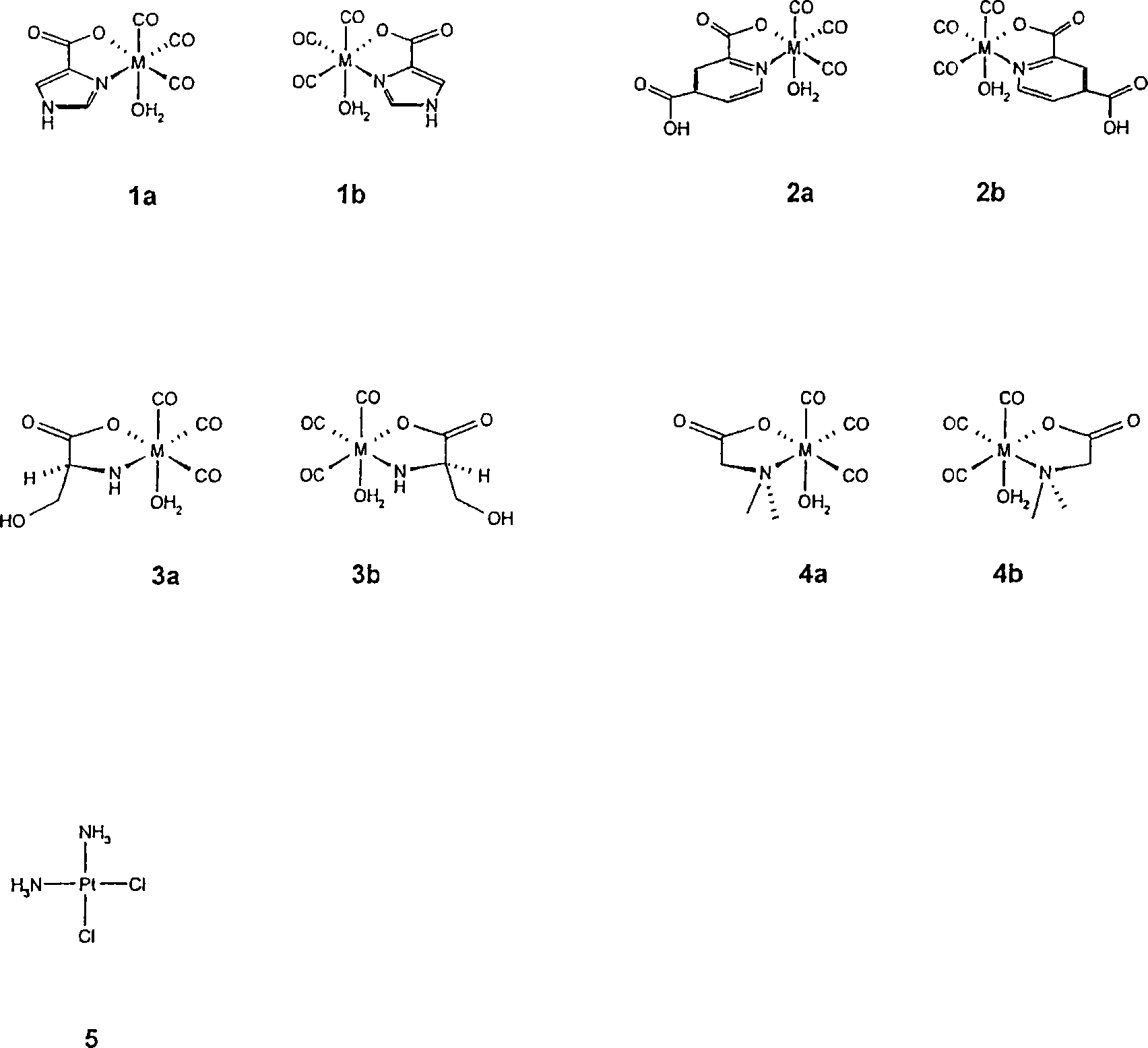
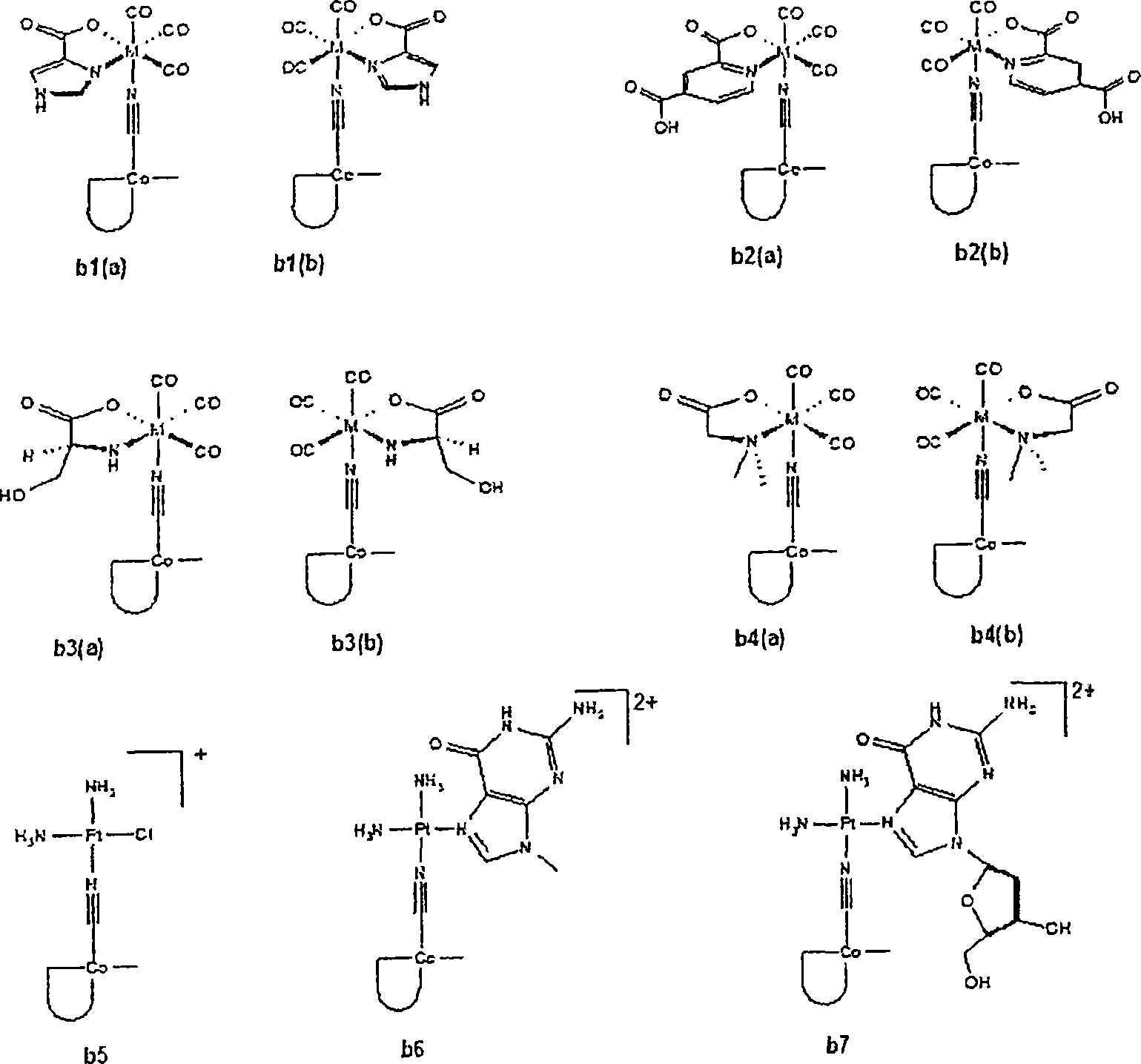
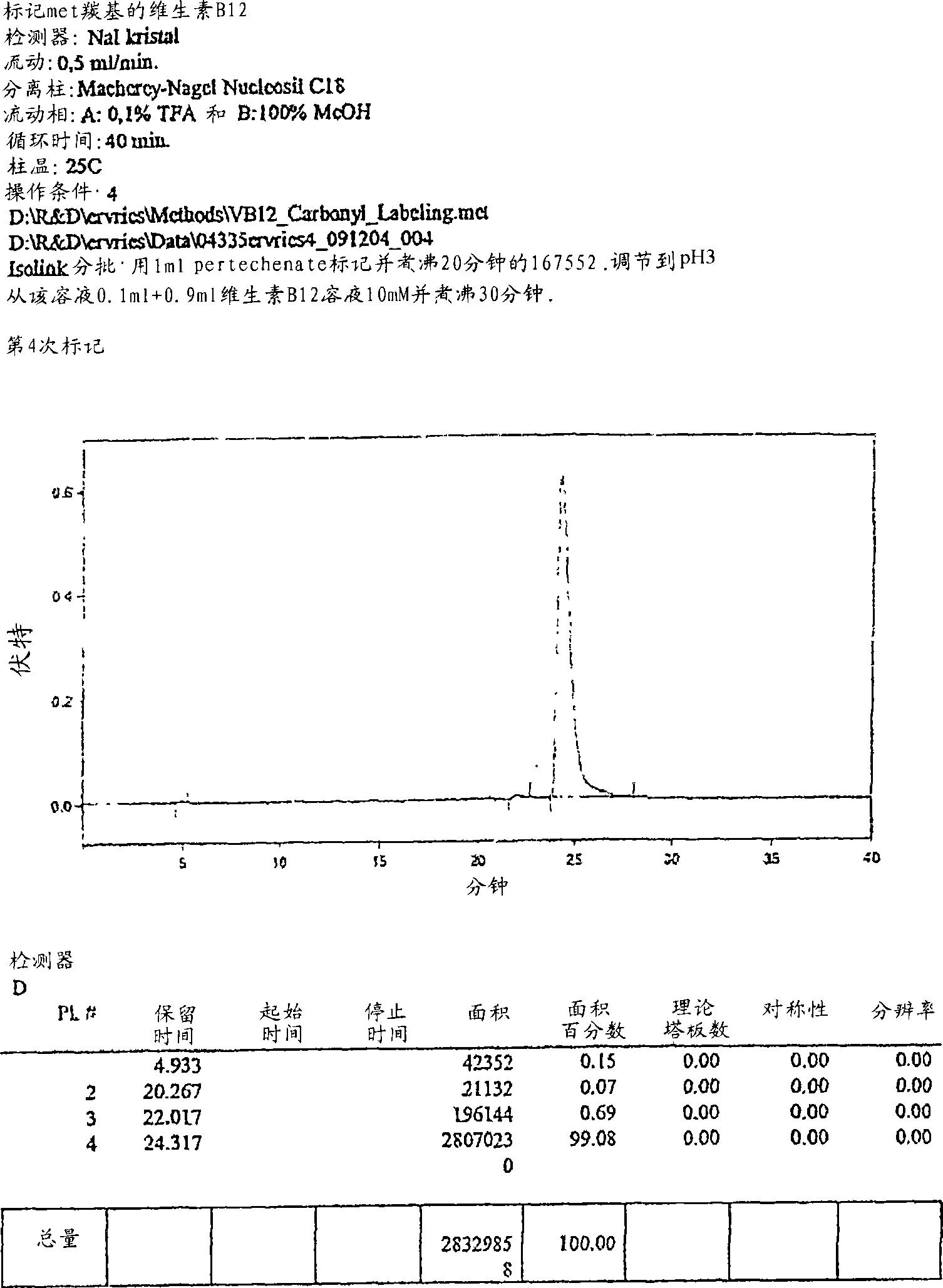
Metal complexes having vitamin B12 as a ligand
A technology of metal complexes and B12, which is applied in pharmaceutical formulations, preparations for in vivo tests, organic chemistry, etc., can solve problems such as inability to be cut and affect the biological behavior of vitamin B, and achieve the effect of simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0060] Materials and methods
[0061] All chemicals were of the highest commercial grade purchased from the companies Merck, Dietikon (CH), Aldrich or Fluka, Buchs (CH) and were used without further purification unless otherwise stated.
[0062] All reactions were performed under nitrogen or argon atmosphere. The reaction was monitored by HPLC.
[0063] HPLC analysis was performed on a Merck-Hitachi L-7000 system equipped with a diode array UV / Vis detector and an EG&G Berthold LB508 radiometric detector. Macherey Nagel Nucleosil C-18ec RP columns (5 μm particle size, 100 Å pore size, 250×3 mm) and Merck C-18e RP Supersphere® columns (100 Å pore size, 250×4 mm) and Waters XTerra RP8 columns (5 μM particle size diameter, 3.0×100mm) for separation. Different HPLC solvent systems and gradients were used: Solvent system 1: 0.1% AcOH and 10% CH in water 3 CN, pH 3 (A) and methanol (B). Solvent system 2: 0.1% triethylammonium acetate and 10% CH in water 3 CN, pH 8. Solvent sys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| absorption coefficient | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com