Fusion albumen containing HSP70 ATPase structure field and mammal p53 albumen and application thereof
A fusion protein, mammalian technology, applied in the field of fusion proteins, can solve the problems of low expression of fusion proteins, difficult to scale industrial production, and amplification, and achieve the effects of less adverse reactions and good immune effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
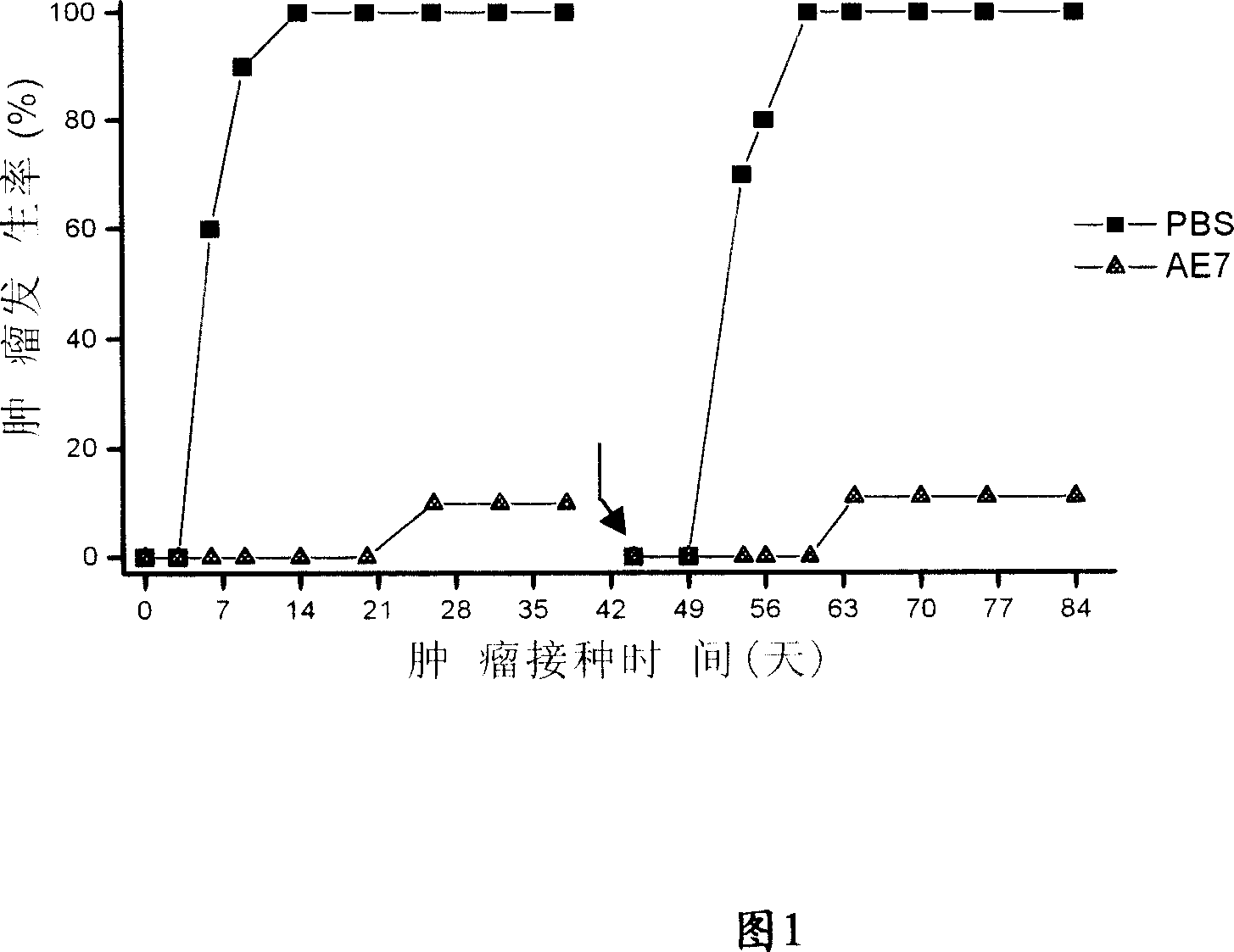
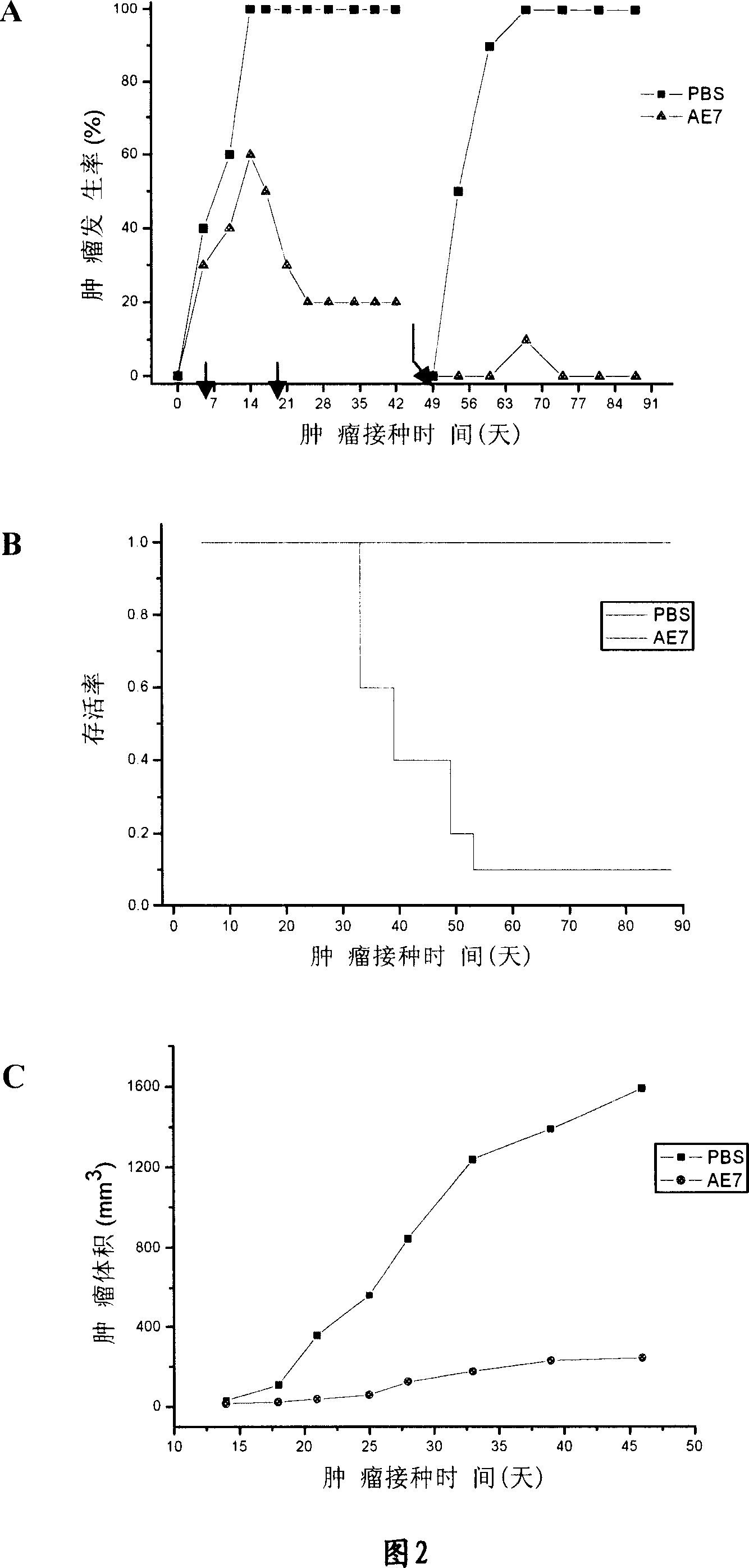
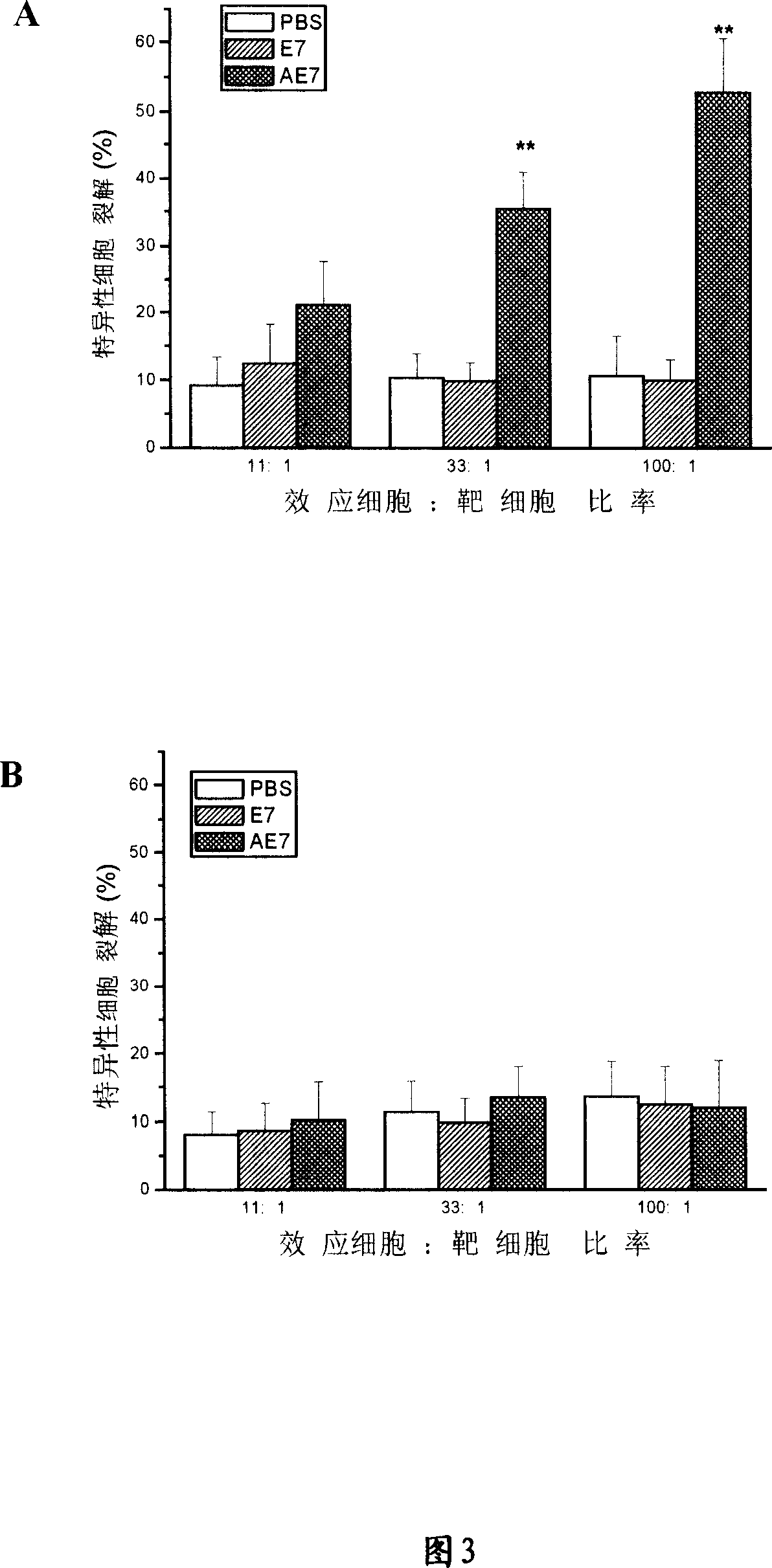
[0030] Example 1. Construction and expression of genes encoding recombinant human HSP70ATPase domain and HPV E7 fusion protein (AE7)
[0031] The E7 and E6 proteins of human papillomavirus (HPV) play an important role in the tumorigenesis of HPV-infected patients (Fehrmann and Laimins, 2003). Therefore, the E7 and E6 proteins on the cell surface of HPV-infected patients can be used as tumor antigens. Recognized by T cells. The fusion protein composed of the ATPase domain of Hsp70 and E7 / E6 can enhance the human body's immune response to HPV virus and cervical cancer caused by it. We call the fusion protein of Hsp70 ATPase domain and E7 AE7, and call the fusion protein of Hsp70 ATPase domain and E6 AE6.
[0032] The human Hsp70 ATPase domain gene (amino acid residues 1-386) was obtained from the full-length human Hsp70 gene by PCR. The HPV virus type 16 E7 gene was obtained by PCR from the virus genome. The PCR product of Hsp70ATPase domain was digested with restriction enzymes Nde...
Embodiment 2
[0038] Example 2. Construction and expression of genes encoding recombinant human HSP70ATPase domain and HPV E6 fusion protein (AE6)
[0039] The HPV virus type 16 E6 gene was obtained by PCR from the virus genome. The PCR product was digested with restriction enzymes BamH I and Xho I and connected to S28bA, named S28bAE6. The AE6 nucleic acid sequence was confirmed by sequencing.
[0040] The S28bAE6 plasmid was transformed into E.coli BL21(DE3) strain for protein expression. The expression method of AE6 protein is the same as that of AE7. The renaturation and purification of AE6 protein is similar to that of AE7. The ATPase activity of AE6 is detected by the malachite green method. The results show that recombinant AE6 has all the functions of hydrolyzing ATP.
Embodiment 3
[0041] Example 3. Construction and expression of genes encoding recombinant human HSP70ATPase domain and human D53 fusion protein (hAP53)
[0042] The human p53 gene was obtained by PCR using a human liver cDNA library as a template. The PCR product of the human p53 gene was digested with restriction enzymes and then ligated to S28bA, named S28bhAP53. The nucleic acid sequence of the target gene was confirmed by DNA sequencing.
[0043] The protein expression, renaturation and purification methods of hAP53 are similar to those of AE7. The ATPase activity is detected by the malachite green method. The results show that the recombinant hAP53 has all the functions of hydrolyzing ATP.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com