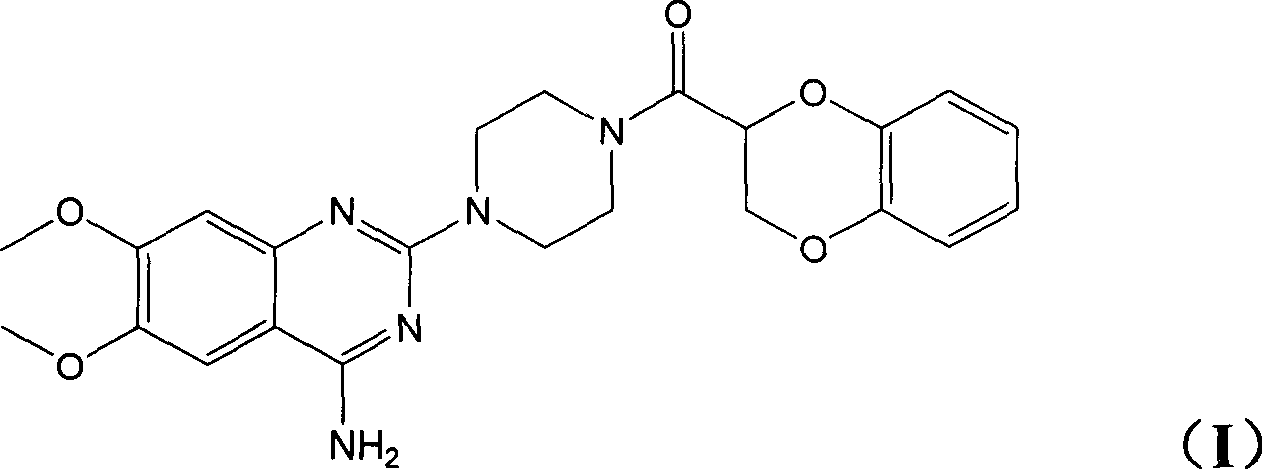
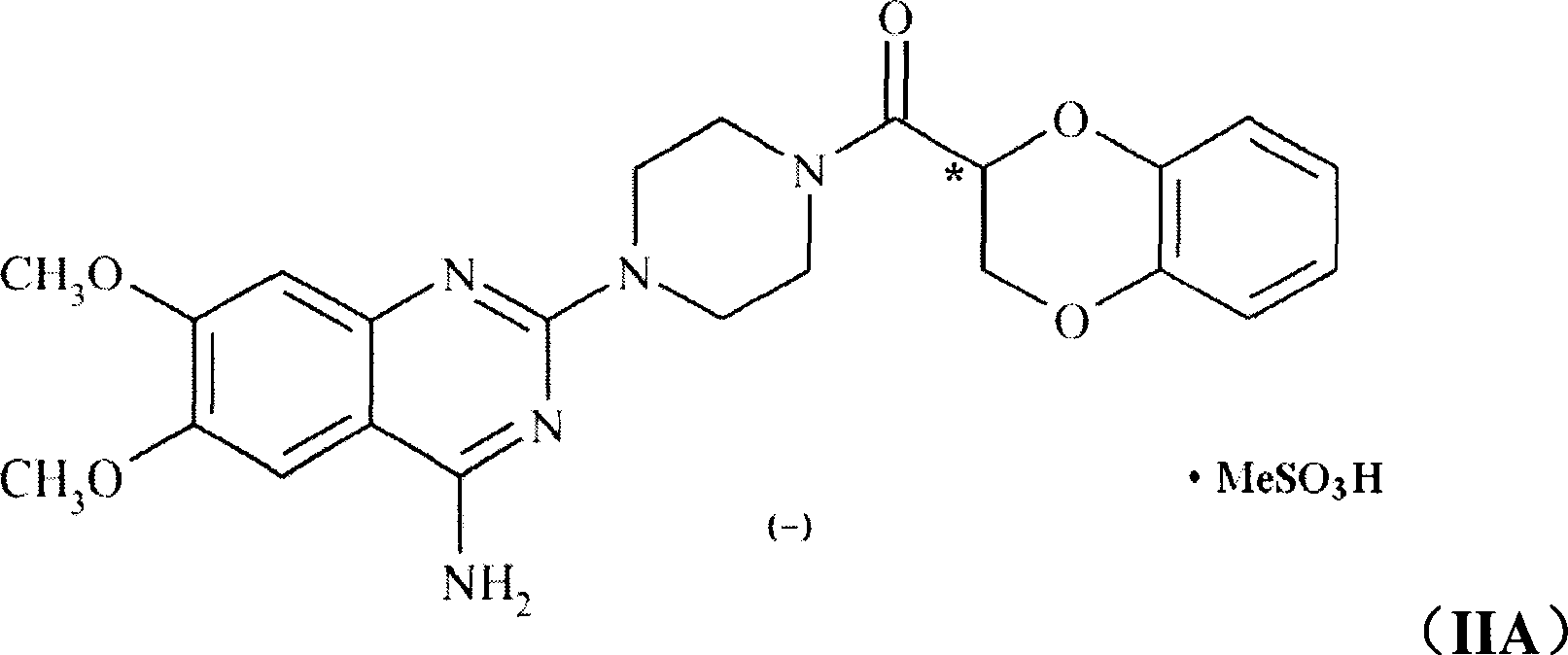
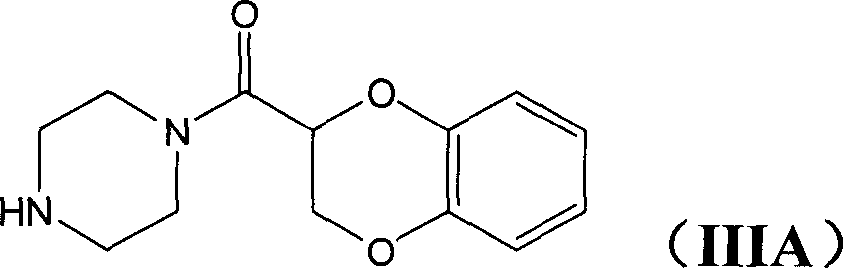
Application of (-)doxazosin in preparing medicine for treating lower urinary tract symptom and bladder excessive activities diseases
A technology of overactive bladder and doxazosin, which is applied in the direction of urinary system diseases, drug combinations, and pharmaceutical formulas, and can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0230] Embodiment 1: Preparation 1 of (-) doxazosin (methanesulfonic acid or hydrochloride) salt tablet
[0231] (-) Doxazosin (methanesulfonic acid or hydrochloric acid) salt 3%
[0232] Lactose 40%
[0233] Starch 33%
[0234] Microcrystalline Cellulose 20%
[0235] Croscarmellose Sodium 3%
[0236] 5% PVP ethanol solution appropriate amount
Embodiment 2
[0238] Embodiment 2: Preparation 2 of (-) doxazosin (methanesulfonic acid or hydrochloride) salt tablet
[0239] (-) doxazosin (methanesulfonic acid or hydrochloride) salt 1%
[0240] Lactose 42%
[0241] Starch 33%
[0242] Microcrystalline Cellulose 20%
[0243] Croscarmellose Sodium 3%
[0244] 5% PVP ethanol solution appropriate amount
[0246] Preparation steps of Examples 1 and 2: (-) doxazosin (methanesulfonic acid or hydrochloride) salt, lactose, microcrystalline cellulose and 1% croscarmellose sodium were sieved and mixed, and mixed with 5 The % PVP ethanol solution is used as a binder to make a soft material, which is granulated, dried, and granulated, and then 2% croscarmellose sodium and magnesium stearate are added, mixed evenly, and compressed into tablets.
Embodiment 3
[0247] Embodiment 3: Preparation 1 of (-) doxazosin (methanesulfonic acid or hydrochloride) salt capsule
[0248] (-) Doxazosin (methanesulfonic acid or hydrochloric acid) salt 3%
[0249] Starch 54%
[0250] Lactose 42%
[0251] 5% PVP ethanol solution appropriate amount
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com