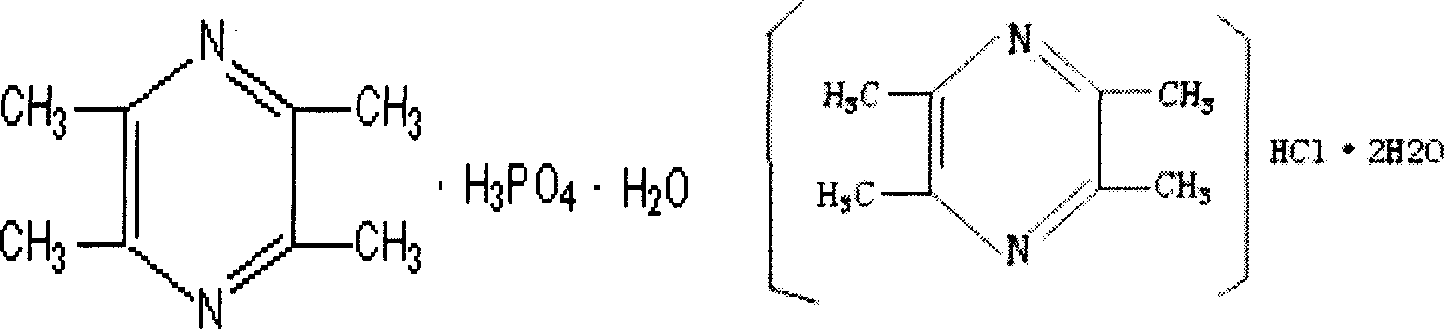
Pharmaceutical composition comprising notoginseng extract, Danshen extract and ligustrazine
A technology of composition and extract, which is applied in the field of medicine to achieve the effects of improved drug efficacy, wide application prospects, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0031] Experimental Example 1 The optimal process for the preparation of Panax notoginseng extract
[0032] Take the medicinal materials of Panax notoginseng, pulverize them into coarse powder, add ethanol for reflux extraction 3 times, 3 hours each time, filter, recover ethanol from the filtrate until there is no alcohol smell, add water to an appropriate amount (each 1 ml is equivalent to 2 g of the original medicinal material), refrigerate for 24 hours, Filtration, adding the filtrate to the treated weakly polar macroporous resin column, first eluting with 2 times the column volume of water, then eluting with 4 to 5 times the 80% ethanol, collecting the eluate, and recovering the ethanol And vacuum drying, that is.
experiment example 2
[0033] Experimental example 2: Determination test of total saponins content of Panax notoginseng in Panax notoginseng extract
[0034] Preparation of reference substance solution Take 10 mg of Panax notoginseng saponins reference substance, vacuum dry at 60°C for 2 hours, accurately weigh, put in a 25ml volumetric flask, add methanol to dissolve and dilute to the mark, shake well, filter with dry filter paper, Precisely measure 5ml of the continuous filtrate, put it in a 25ml volumetric flask, add methanol to dilute to the mark, and shake well.
[0035] Preparation of the test solution: Take 15mg of this product, accurately weigh it, put it in a 25ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well, filter through dry filter paper, accurately measure 5ml of the subsequent filtrate, and place it in a 25ml measuring bottle , add methanol to dilute to the mark, shake well, and get it.
[0036] Determination method Precisely measure 1ml of the test so...
experiment example 3
[0039] Experimental Example 3 Salvia Extraction Technology Screening Test
[0040] 1. Water extraction process screening test
[0041] The amount of water, extraction time and extraction times have great influence on the extraction results. Therefore, taking the total phenolic acid content of Salvia miltiorrhiza and the extract yield as indicators, the influence of three factors on the extraction results was investigated by orthogonal test method. The factors and level design are shown in Table 2.
[0042] Level / Factor
[0043] Test method Weigh 100g of Salvia miltiorrhiza, a total of 9 parts, extract according to the requirements of each column number in Table 2, combine the extracts, filter, concentrate the filtrate and dilute to 500ml, for use.
[0044] Determination of total phenolic acids in dandelion
[0045] Preparation of reference solution Precisely weigh 1 mg of dry protocatechualdehyde reference substance, dissolve in water, and make up to 100 ml.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com