Method for improving conversion rate of oil soluble unsaturated lipids into water-soluble lipids
An unsaturated and water-soluble technology, applied in chemical instruments and methods, liposome delivery, edible oil/fat, etc., can solve problems such as reducing the safety and stability of oil-soluble fatty acids, damaging harmful substances, and stimulating the human body. To achieve the effect of increasing absorption, reducing toxicity, and promoting absorption in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0042] Embodiment 1: Method for preparing water-soluble lipoplexes from fatty acids using catalysts
[0043] 1. Preparation of Conjugated Linoleic Acid-Chloride Derivatives
[0044] 100 g of conjugated linoleic acid (80%) was added to 50 ml of pyridine for complete dissolution, and 50 ml of thionyl chloride was added to the resulting solution by split injection at 4°C. If thionyl chloride is not added by split injection, the reaction proceeds rapidly and rancidity of the conjugated linoleic acid cannot be avoided. To the resulting solution was added thionyl chloride by split injection and stirred for about 40 minutes resulting in the formation of crystals. The resulting solution containing these crystals was allowed to stand at room temperature for about two hours, and then filtered to obtain the conjugated linoleic acid-chloride derivative in the form of crystals.
[0045] 2. Preparation of Glycine-Conjugated Linoleic Acid Complex
[0046] 100 g of conjugated linoleic acid...
Embodiment approach 2
[0048] Embodiment 2: Method for preparing water-soluble lipoplexes from fatty acids using catalysts
[0049] 1. Preparation of DHA-chloride derivatives
[0050] DHA was substituted for conjugated linoleic acid in the same manner as in Step 1 of Embodiment 1 to obtain a DHA-chloride derivative in the form of crystals.
[0051] 2. Preparation of Lysine-DHA Complex
[0052] In step 2 of embodiment 1, glycine is replaced by lysine, and 3 ml instead of 5 ml of pyridine and thionyl chloride are added to the resulting solution in the same manner as in step 2 of embodiment 1 in a ratio of 1:1 to form DHA-chloride derivative, thus collecting 139 g of water-soluble complex.
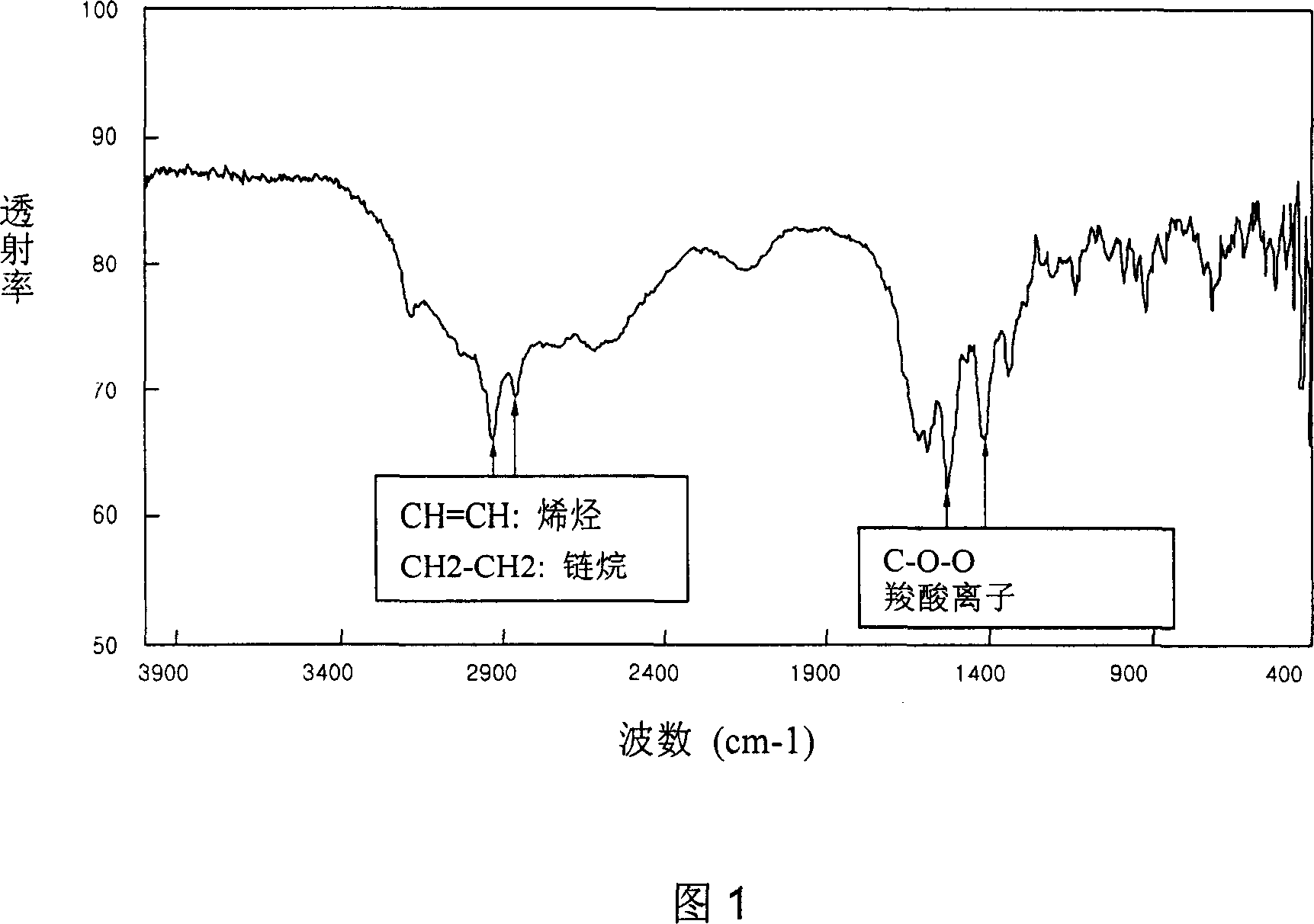
[0053] The water-soluble complex obtained in the above steps was detected with an infrared analyzer, and the results are shown in Figure 1.
[0054] As shown in Figure 1, the carboxylate ion (COO - ) peak is shown at 1560cm -1 and 1395cm -1 .
Embodiment approach 3
[0055] Embodiment 3: Another method of preparing water-soluble lipoplexes from fatty acids using emulsifiers
[0056] Stir 200 mg of conjugated linoleic acid and 1 L of water at a stirring rate of 5,000 rpm, keep the temperature at 4° C., then add 2.0% by weight of lecithin and 2.0% by weight of sucrose fatty acid ester to the resulting solution based on the total weight of the solution. and 0.05% by weight of ascorbic acid emulsifier to emulsify. Then, a mixed powder of 100 g of glycine and 100 g of lysine was slowly added to the reactant, and the reaction conditions of 4° C. and 5,000 rpm were maintained until the reactant became homogeneous. At the point where the resulting solution became clear, the reaction was terminated. As a result, 393 g of a water-soluble lipoplex was obtained using a freeze dryer.
[0057] The water-soluble complex obtained in the above process was detected with an infrared analyzer, and the results are shown in FIG. 1 .
[0058] As shown in Figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com