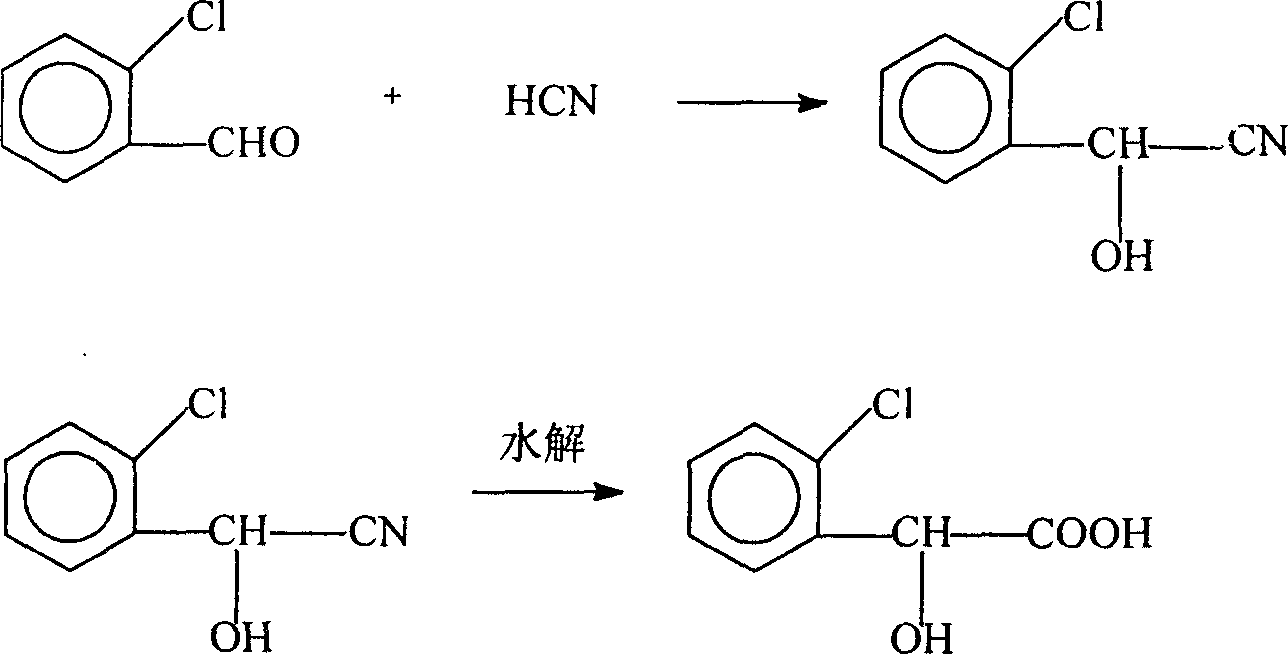
Process for preparing DL-ortho-chloro mandelic acid
A technology of o-chloromandelic acid and o-chlorobenzaldehyde, applied in the field of preparation of DL-o-chloromandelic acid, can solve the problems of high production cost and low yield, and achieve the effect of increasing yield and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In the 250ml three flasks that have thermometer, condensing tube, agitator, add 42.9 grams of o-chlorobenzaldehyde (mass percentage content is 98.5%), water 6 grams, catalyst sodium hydroxide 5.7 grams (mass percentage content is 5%) , at a temperature of -5°C, add 10.1 grams of aqueous hydrocyanic acid solution (80% by mass) dropwise within 30 minutes. gram (mass percentage content is 31%) is hydrolyzed, and after dropwise addition, after insulation reaction 1 hour, add 240ml toluene, heat up and make toluene and water azeotropic dehydration, ammonium chloride is separated out in the reaction solution, and filter removes ammonium chloride, filtrate Cooling, crystallization, filtration to get the crude product DL-o-chloromandelic acid, the crude product DL-o-chloromandelic acid was dissolved in 360ml toluene and recrystallized to obtain 39.5 grams of qualified white DL-o-chloromandelic acid crystals, content 99.2%, melting point 82.8~ 83.5°C, yield 67%.
Embodiment 2
[0027] Carry out feeding operation by embodiment 1, difference is that the product recrystallization mother liquor among the example 1 is used as dehydrating agent. The crude product DL-o-chloromandelic acid was dissolved in 380ml of toluene for recrystallization to obtain 40.6 grams of qualified DL-o-chloromandelic acid with a content of 99.3%, a melting point of 83-83.6°C, and a yield of 72%.
Embodiment 3
[0029] Carry out feeding operation by embodiment 1, difference is that 10.1 gram mass percent content in example 1 is that 80% hydrocyanic acid aqueous solution is changed into 30 gram mass percent content and is 30% hydrocyanic acid aqueous solution, obtains qualified DL-o Chloromandelic acid 40.1 g, content 99.2%, melting point 82.9-83.5°C, yield 71.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com