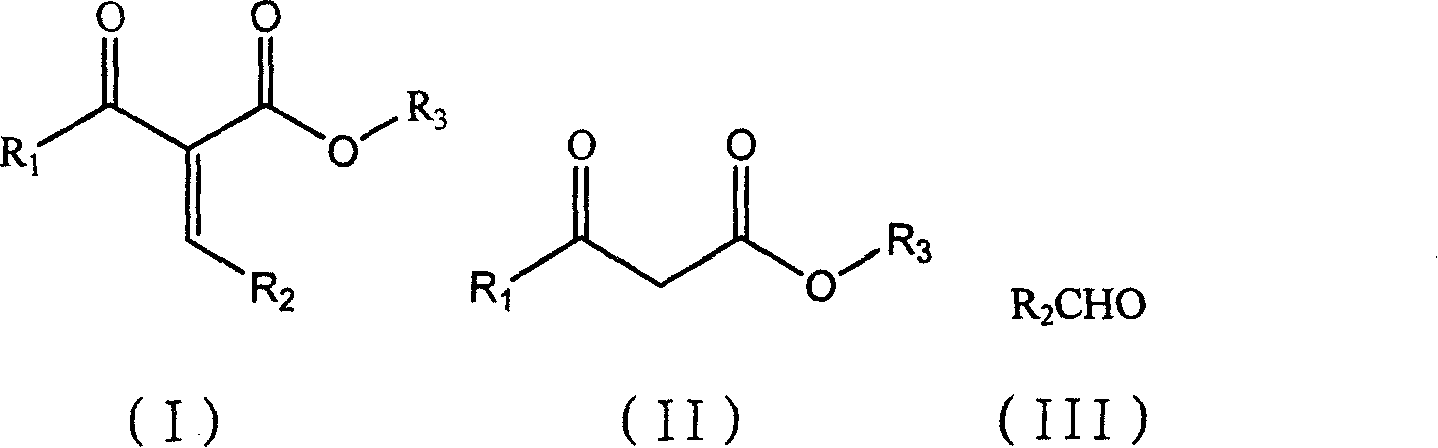
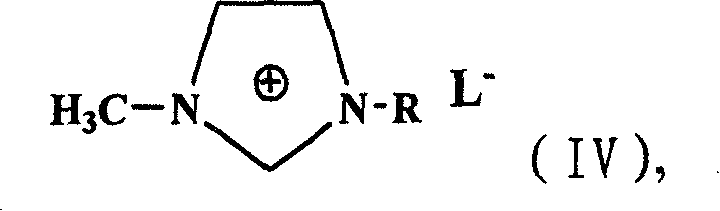
Preparation method of alpha-acetyl substituted alpha, beta-unsaturated ester
An acyl-substituted and unsaturated technology, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as difficult to meet environmental friendliness and environmental pollution, and achieve non-flammable and explosive safety , less environmental pollution, easy to operate and handle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthesis of embodiment 1α-acetyl group-beta-phenyl acrylate ethyl ester
[0020] Add ethyl acetoacetate (13g, 0.10mol), benzaldehyde (10.6g, 0.1mol), proline (10mmol) and 1-butyl-3-methylimidazolium tetrafluoroboron successively in a 100mL single-necked round bottom flask Salt ionic liquid 10ml, stirred at room temperature for 12h, stopped the reaction with saturated ammonium chloride solution, extracted with ethyl acetate, separated the organic layer, separated and purified to obtain 18.5g of the product, with a yield of 85%. The aqueous layer was extracted with dichloromethane, and the solvent was evaporated to obtain 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, which was recycled for use.
Embodiment 2
[0021] The synthesis of embodiment 2α-acetyl group-β-phenyl acrylate ethyl ester
[0022] Add ethyl acetoacetate (13g, 0.10mol), benzaldehyde (10.6g, 0.1mol), glycine (10mmol) and 1-butyl-3-methylimidazole tetrafluoroborate successively in a 100mL single-necked round bottom flask 10ml of ionic liquid was stirred and reacted at room temperature for 10h. The reaction was terminated with saturated ammonium chloride solution, extracted with ethyl acetate, and the organic layer was separated, separated and purified to obtain 20.1g of the product with a yield of 92.2%. The aqueous layer was extracted with dichloromethane, and the solvent was evaporated to obtain 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, which was recycled for use.
Embodiment 3
[0023] The synthesis of embodiment 3α-acetyl group-beta-phenyl acrylate ethyl ester
[0024] Add ethyl acetoacetate (13g, 0.10mol), benzaldehyde (10.6g, 0.1mol), threonine (10mmol) and 1-butyl-3-methylimidazolium tetrafluoroboron successively in a 100mL single-necked round bottom flask Salt ionic liquid 10ml, stirred at room temperature for 14h, stopped the reaction with saturated ammonium chloride solution, extracted with ethyl acetate, separated the organic layer, separated and purified to obtain 19.2g of the product, with a yield of 88%. The aqueous layer was extracted with dichloromethane, and the solvent was evaporated to obtain 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid, which was recycled for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com