Preparation method of 1,4-dipregnene-16-beta-methyl-17-alpha-21-bihydroxy object
A technology of pregnane and hydroxy substances, applied in the direction of steroids and organic chemistry, can solve the problems of difficult separation of products and by-products, inconvenient follow-up reactions, complex processes, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
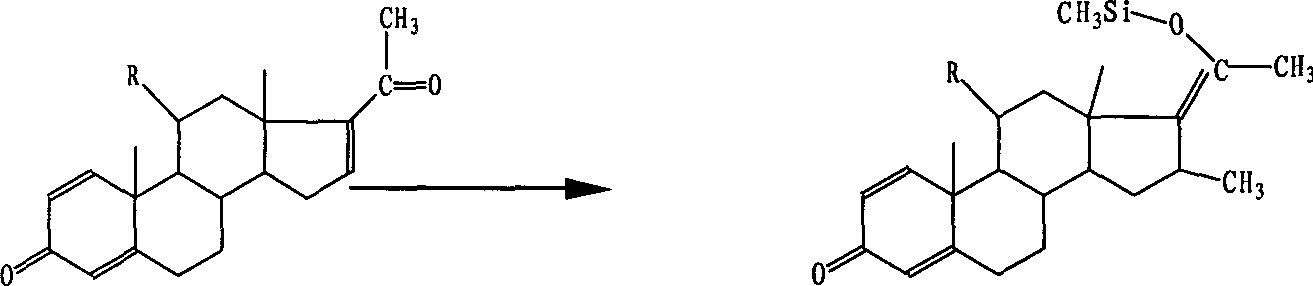
[0021] 1) 1,4,16-gestrinene and cuprous bromide dimethyl sulfide complex of 0.025 times the amount of substance, trimethylchlorosilane of 1 times the amount of substance, chlorine of 1 times the amount of substance Methylmagnesium and 2 times the amount of hexamethyl
[0022] Triamine phosphate is subjected to Grignard reaction in tetrahydrofuran at a reaction temperature of -50 to -40°C and a reaction time of 3 hours to obtain a Grignard compound;
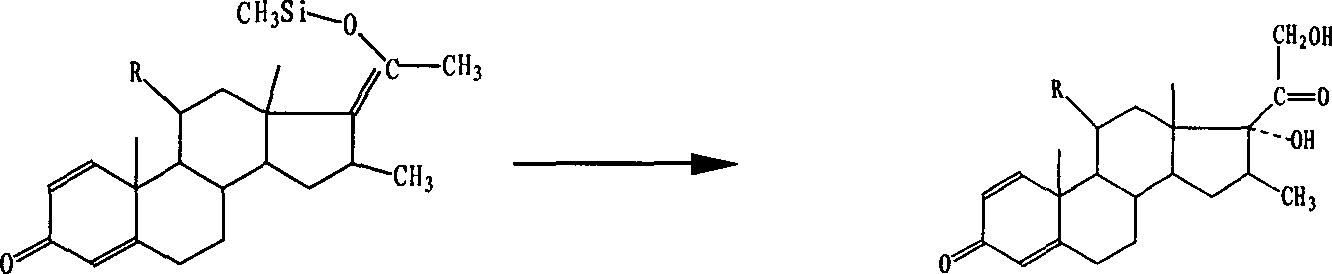
[0023] 2) React the above-mentioned Grignard substance with potassium bicarbonate of 1 times the amount of the substance and m-chloroperoxybenzoic acid of 1 times the amount of the substance in dichloromethane, the reaction temperature is -30~-25 ° C, and the reaction time is 1 hours; the pH value of the solution was adjusted to 1 with hydrochloric acid to obtain the product 1,4-pregnane-16β-methyl-17α,21-dihydroxy, with a total yield of 81.4%.
Embodiment 2
[0025] 1) 1,4,16-gestrinene and cuprous bromide of 0.1 times of substance amount, trimethylchlorosilane of 4 times of substance amount, methylmagnesium bromide of 1.5 times of substance amount and 4 Hexamethyltriamine phosphate in an amount twice the amount of the substance is subjected to a Grignard reaction in ether at a reaction temperature of -15 to -10°C and a reaction time of 15 hours to obtain a Grignard substance;
[0026] 2) react the above-mentioned Grignard substance with sodium bicarbonate of 10 times the amount of the substance, and m-chloroperoxybenzoic acid of 5 times the amount of the substance in chloroform, the reaction temperature is -10~-5°C, and the reaction time is 5 hours; The pH value of the solution was adjusted to 4 with hydrochloric acid to obtain the product 1,4-pregna-16β-methyl-17α,21-dihydroxy. The overall yield is 76%.
Embodiment 3
[0028] 1) Mix 1,4,16-gestrinene with cuprous chloride of 0.05 times the amount of the substance, trimethylchlorosilane of 1 to 4 times the amount of the substance, and methylmagnesium iodide of 1.25 times the amount of the substance and three times the amount of hexamethylphosphoric acid triamine in tetrahydrofuran for Grignard reaction, the reaction temperature is -30 ~ -25 ° C, the reaction time is 9 hours, to obtain the Grignard substance;
[0029] 2) React the above-mentioned Grignard substance with potassium carbonate of 5 times the amount of the substance and m-chloroperoxybenzoic acid of 4.5 times the amount of the substance in dichloroethane, the reaction temperature is -30~-25 ° C, and the reaction time is 3.5 hours; the pH value of the solution was adjusted to 3 with sulfuric acid to obtain the product 1,4-pregnane-16β-methyl-17α,21-dihydroxy. The overall yield is 78.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com