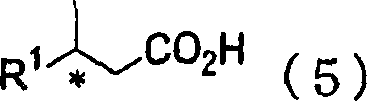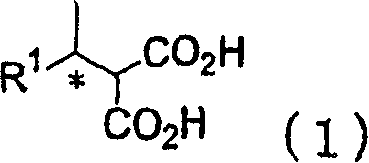Process for producing optically active alcohol and carboxylic acid
An optically active and optically pure technology, applied in the field of preparation of optically active alcohols and carboxylic acids, can solve the problems of a large number of solvents, low efficiency, long time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0229] Embodiment A (use microorganism to prepare the alcohol with optical activity)
[0230] (1) Isolation of microorganisms that produce (S)-2-pentanol from 2-pentanone and microorganisms that produce (S)-2-hexanol from 2-hexanone
[0231] Liquid containing 5g / L yeast extract (manufactured by Difco), 5g / L polypeptone (manufactured by Nippon Pharma), 3g / L malt extract (manufactured by Difco), 20g / L glucose (manufactured by Nippon Food Processing) in 2.5mL Various bacterial strains shown in Table 1 were inoculated in the culture medium, and aerobically cultured at 30° C. for 24-72 hours. 1 mL of the culture solution was taken from each of the obtained culture solutions, and bacterial cells were collected by centrifugation. Add 0.04mL Tris-HCl buffer solution (pH 7.0) and 9.028mL desalted water to the cells to fully suspend the cells, then add 0.05mL 100g / L glucose, 9.02mL 12g / L NADP + (manufactured by Oriental Yeast), and 0.01 mL of the reaction substrate 2-pentanone or 2-he...
Embodiment B
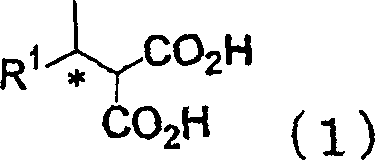
[0301] Embodiment B (preparation of optically active carboxylic acid by chemical synthesis)
Embodiment 1
[0302] (Embodiment 1) Synthesis of (S)-2-methanesulfonyloxypentane
[0303] Into a 200 mL three-necked flask were added 4.14 g (47.0 mmol, 99.1% e.e.) of (S)-2-pentanol and 9.8 mL (71 mmol) of triethylamine, 41 mL of dichloromethane. The mixture was ice-cooled, and 4.36 mL (56.4 mmol) of methanesulfonyl chloride was added dropwise. After stirring for 30 minutes, 40 mL of saturated ammonium chloride aqueous solution and 20 mL of water were added to terminate the reaction. The mixture was extracted with 80 mL of diethyl ether, and the organic layer was washed with 20 mL of saturated aqueous ammonium chloride solution and 20 mL of saturated brine, and dried over magnesium sulfate. The solvent was distilled off to obtain 8.5 g of crude (S)-2-methanesulfonyloxypentane, which was used in the following reaction without further purification.
[0304] 1 H-NMR (400MHz, CDCl 3 )δ0.95(t, J=7.2Hz, 3H), 1.42(d, J=6.3Hz, 3H), 1.34-1.50(m, 2H), 1.50-1.63(m, 1H), 1.67-1.77(m , 1H), 3.00(s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Optical purity | aaaaa | aaaaa |
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com