Process for preparing solvent orange 2A
A production process and technology of solvent orange, applied in the field of production process of solvent orange 2A, can solve the problems of long reaction time, large amount of solvent and high production cost, and achieve the effects of high equipment utilization rate, reduced reaction time and strong polarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
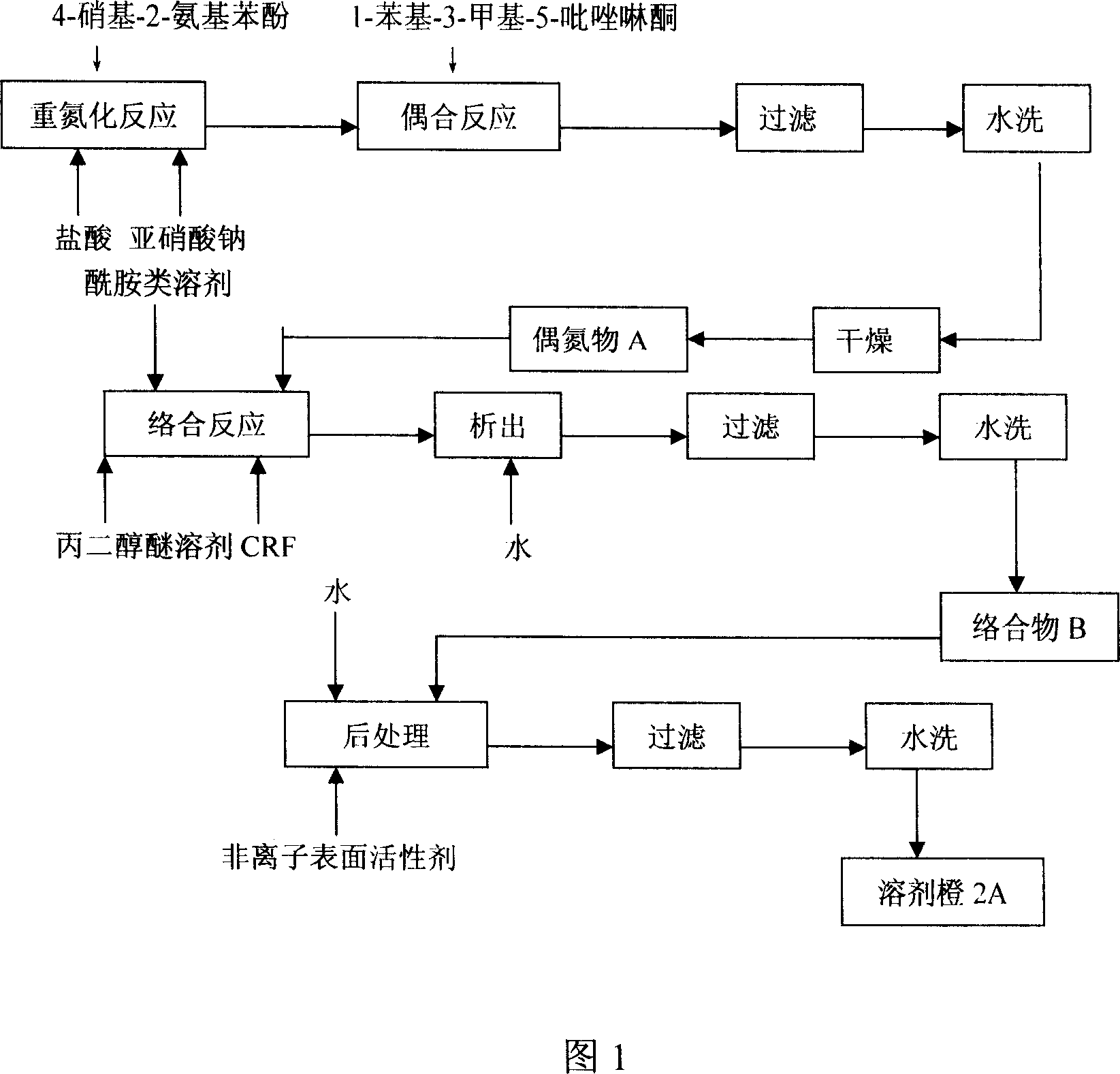
[0036] Embodiment 1: according to the processing steps shown in Fig. 1.
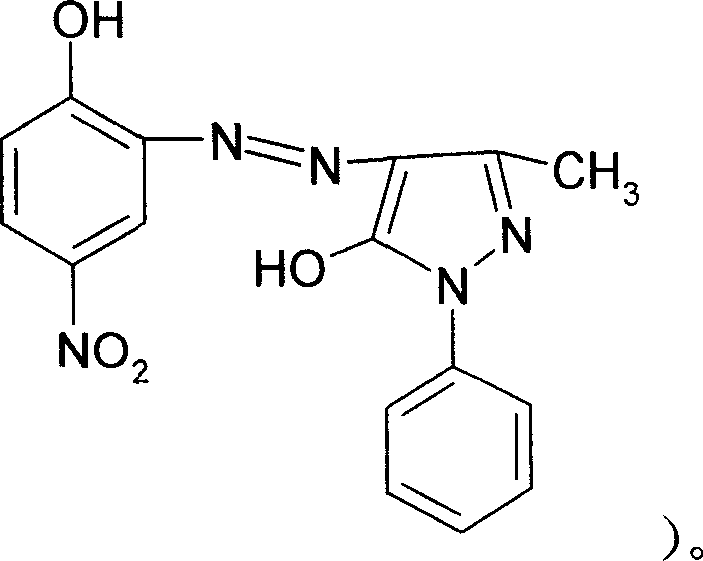
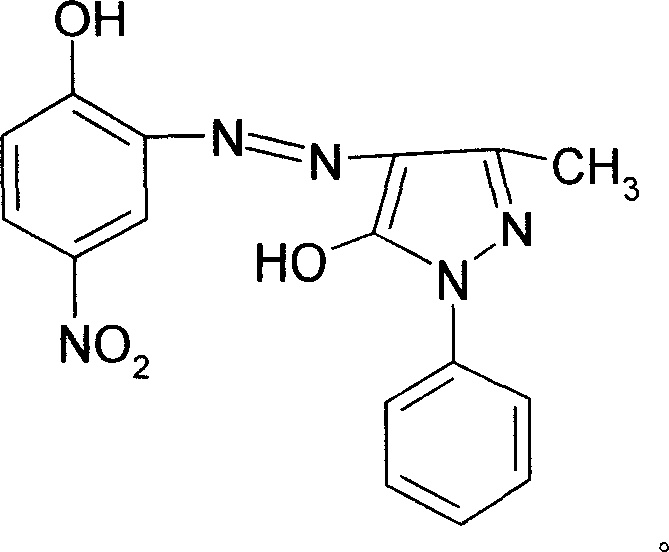
[0037] 1. Coupling reaction: After diazotization reaction, 350 kg of 4-nitro-2-aminophenol is coupled with 360 kg of 1-phenyl-3-methyl-5-pyrazolone, and the product is filtered, washed and dried After that, the intermediate product azo compound A is obtained.
[0038] 2. Complexation reaction: 600 kilograms of mixed solvents prepared by dimethylformamide and formamide in a weight ratio of 4:1 are added to 100 kilograms of propylene glycol butyl ether solvents and 100 kilograms of complexing agent CRF (Cr 3 (HCOO) X (OH) Y , X+Y=9), heated to 100°C, added azo compound A, directly heated to 138-150°C without reflux, reacted for 3 hours, precipitated into water after cooling down, filtered, and washed with water to obtain complex B.
[0039] 3. Substitution reaction: beat complex B in water, add 250 kg of nonionic surfactant R-NH 2 Cl (R is an alkyl group with 10-12 carbon atoms), heated to 60°C, reacte...
Embodiment 2
[0041] 1. Coupling reaction: After diazotization reaction, 340 kg of 4-nitro-2-aminophenol was coupled with 350 kg of 1-phenyl-3-methyl-5-pyrazolone, and the product was filtered, washed and dried After that, the intermediate product azo compound A is obtained.
[0042] Two, complexation reaction: 583 kilograms of mixed solvents prepared by dimethylformamide and formamide in a weight ratio of 3.5:1 are added to 97 kilograms of propylene glycol ether and 90 kilograms of complexing agent CRF (Cr 3 (HCOO) X (OH) Y , X+Y=9), heated to 100°C, added azo compound A, directly heated to 138-150°C without reflux, reacted for 2.8 hours, precipitated into water after cooling down, filtered, and washed with water to obtain complex B.
[0043] 3. Substitution reaction: beat complex B in water, add 230 kg of nonionic surfactant R-NH 2 Cl (R is an alkyl group with 10 carbon atoms), heated to 60°C, reacted for 4 hours, filtered, washed with water, and dried to obtain 1000 kg of Solvent Oran...
Embodiment 3
[0045]1. Coupling reaction: After diazotization reaction, 370 kg of 4-nitro-2-aminophenol was coupled with 375 kg of 1-phenyl-3-methyl-5-pyrazolone, and the product was filtered, washed and dried After that, the intermediate product azo compound A is obtained.
[0046] 2. Complexation reaction: 617 kilograms of mixed solvents prepared by dimethylformamide and formamide in a weight ratio of 4.3:1 are added to 103 kilograms of propylene glycol methyl ether and 110 kilograms of complexing agent CRF (Cr 3 (HCOO) X (OH) Y , X+Y=9), heated to 100°C, added azo compound A, directly raised the temperature to 138-150°C without reflux, reacted for 3.5 hours, precipitated into water after cooling down, filtered, and washed with water to obtain complex B.
[0047] 3. Substitution reaction: beat complex B in water, add 260 kg of nonionic surfactant R-NH 2 Cl (R is an alkyl group with 12 carbon atoms), heated to 60°C, reacted for 4 hours, filtered, washed with water, and dried to obtain 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com