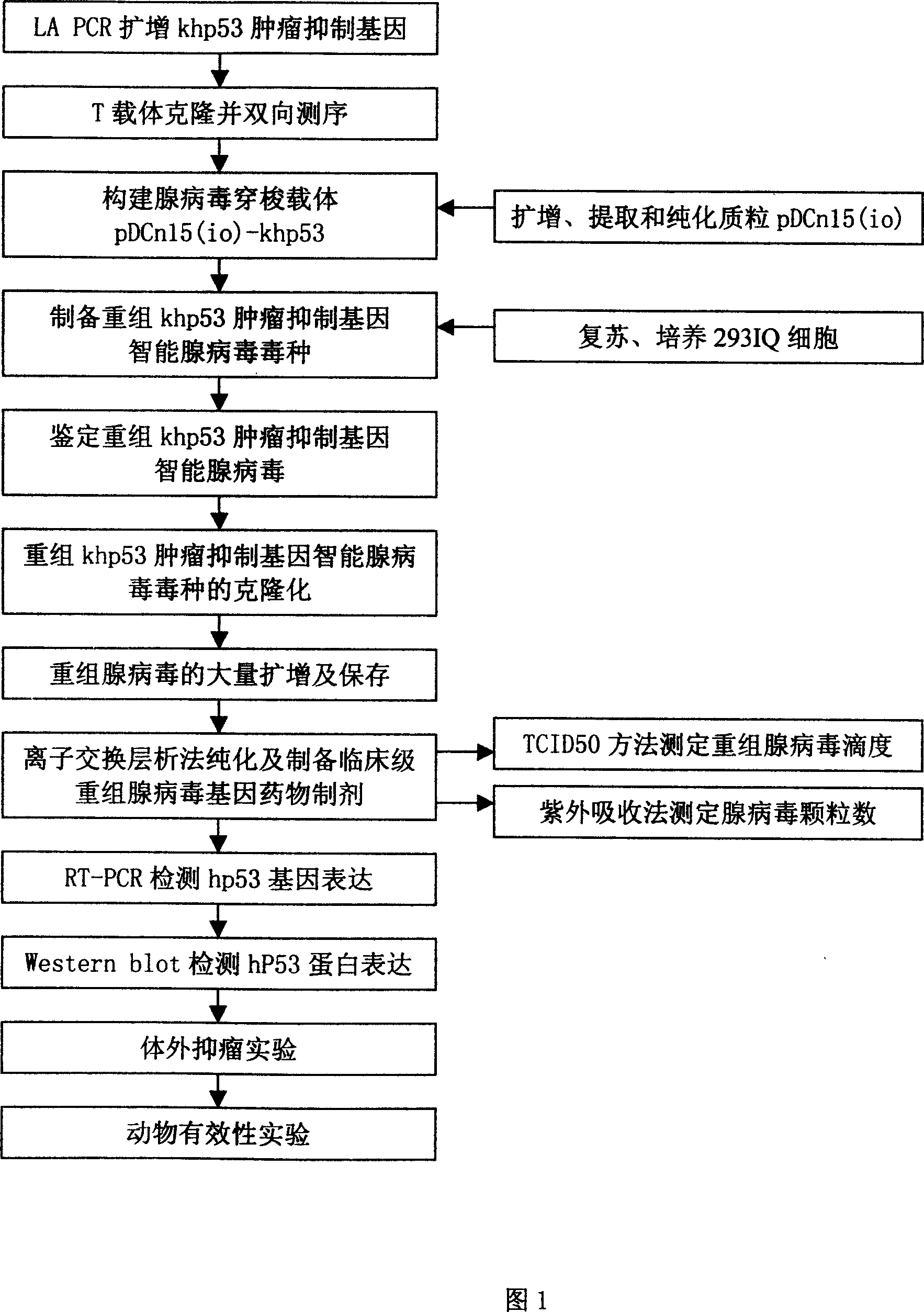
Recombinant of intelligent adenovirus vector and khp53 gene and application thereof
A technology of p53 gene and viral vector, which is applied in the field of genetic engineering, can solve problems such as difficult to pre-estimate the titer and yield of adenoviral vector, and achieve the effect of wide host range, good safety and low pathogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Construction of recombinant khp53 tumor suppressor gene smart adenovirus
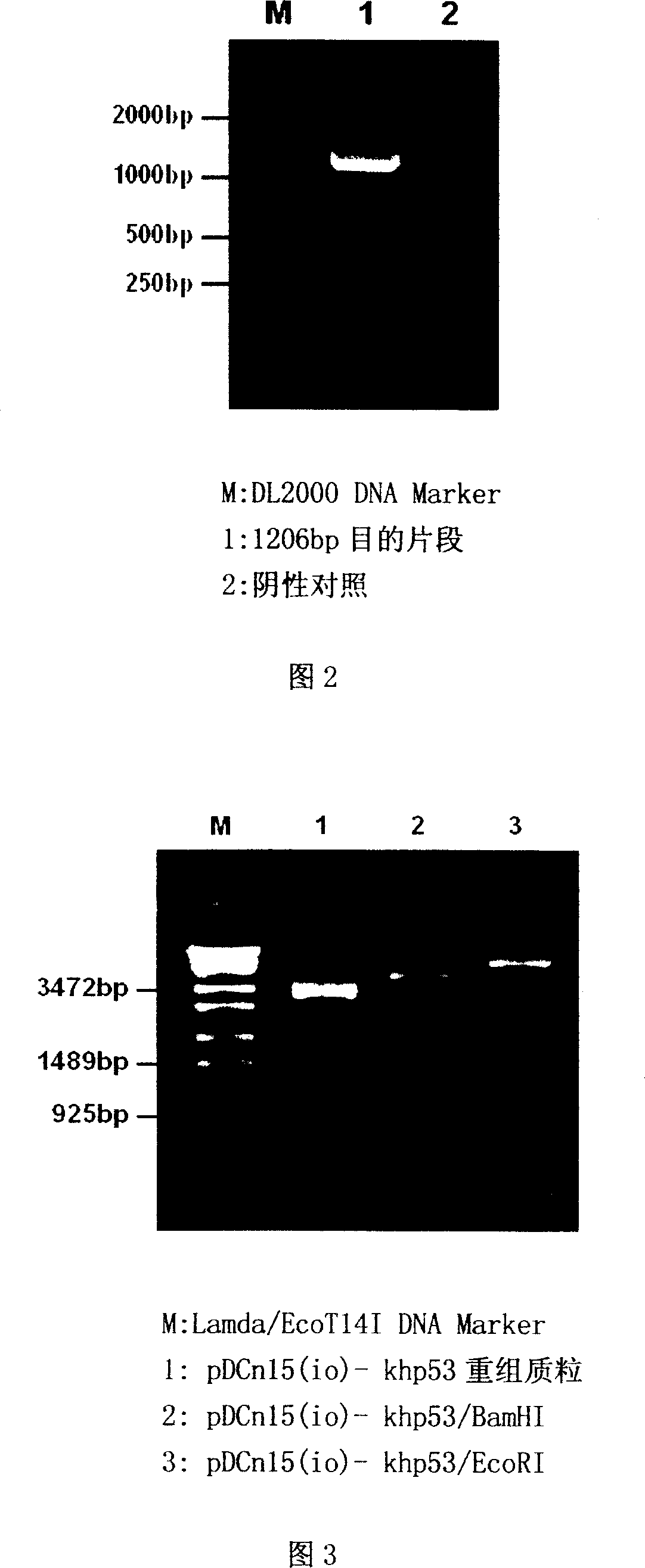
[0041] 1) Acquisition of the coding sequence of human p53 tumor suppressor gene with Kozak rule sequence (abbreviated as khp53): According to the published full sequence of human wild-type p53 cDNA, two primers were designed and synthesized, the upstream primer 5'-ATA GGATCC A CCATGGAGG AGC CGC AGT C 3', downstream primer 5'-ATA GGATCC ATG TCA GTC TGA GTC AG 3', (both upstream and downstream primers insert the BamHI restriction site GGATCC and restriction base ATA, and the upstream primer BamHI enzyme An A base is added after the cutting site GGATCC, and finally the sequence after the first two bases CC of the start codon ATG in the human p53 cDNA, that is, CCATGG AGG AGC CGC AGT C, so that the BamHI restriction site GGATCC The CC base, the added A base and the own CCATGG in the human p53 cDNA constitute the Kozak regular sequence CCACCATGG), using the human p53 cDNA as a template, usi...
Embodiment 2
[0045] Example 2: Identification, cloning, massive amplification, purification and preparation of clinical-grade gene drug preparations of recombinant khp53 tumor suppressor gene intelligent adenovirus
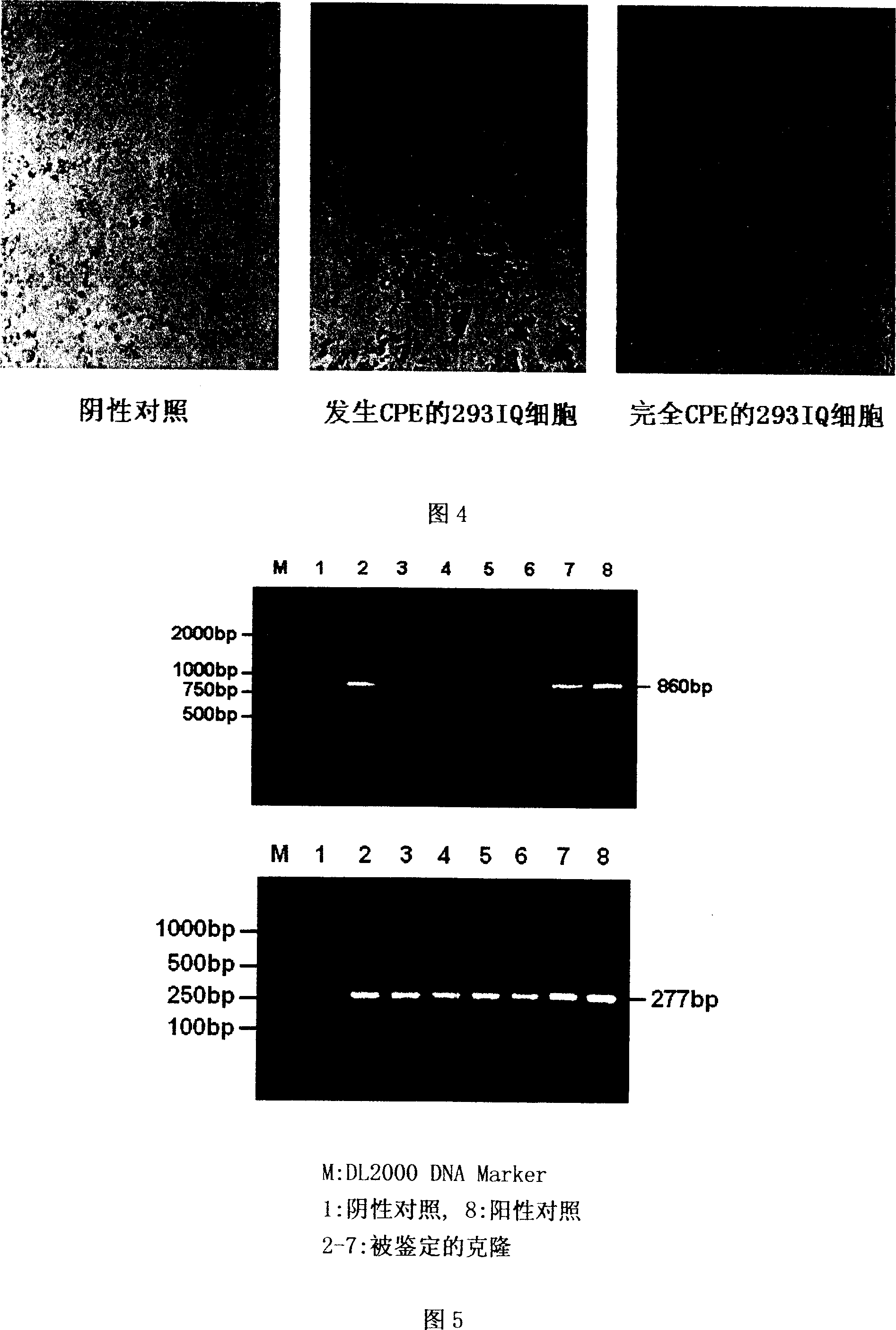
[0046] 1) Identification of recombinant khp53 tumor suppressor gene smart adenovirus: PCR amplification of Ad5 genome with upstream primer: 5'-TCG TTT CTC AGC AGCTGT TG 3', downstream primer: 5'-CAT CTG AAC TCA AAG CGT GG 3' Specific fragment (11-13.4mu, 860bp) to identify whether the produced virus is type 5 adenovirus; use upstream primer 5'-AGCACTGTCC AACAACACCA 3', downstream primer 5'-ATA GGATCC ATG TCA GTC TGA GTC AG 3', PCR Amplify the specific fragment of 277bp in the coding region of the human p53 tumor suppressor gene to identify whether the human p53 tumor suppressor gene is inserted into the type 5 adenovirus. The results are shown in Figure 5, a total of 6 clones, all of which are type 5 adenovirus And both carry the human p53 tumor suppressor gene.
[0047] 2) C...
Embodiment 3
[0050] Example 3: Titer determination of recombinant khp53 tumor suppressor gene smart adenovirus, determination of the number of adenovirus particles and gene expression analysis in human malignant tumor cells
[0051] 1) The TCID50 method was used to determine the titer of recombinant adenovirus: 293IQ cells were prepared with MEM culture medium at a concentration of 1×10 5 The suspension of cells / ml was inoculated in 96-well plate at 100 μl / well; at the same time, virus dilutions were prepared according to 10-fold dilution to infect the above cells respectively, and 100 μl of the same concentration of virus dilutions were added to the first 10 wells of each row. An equal volume of 2% BCS MEM was added to 12 wells as a negative control. 37°C, CO 2 Cultivate in the incubator for 10 days, observe under an inverted microscope, judge and record the CPE situation, and finally calculate the titer of the recombinant adenovirus according to the formula, T=10 1+d(S-0.5) IU / ml, wher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com