1-methylamino-1- methylthio-2-nitroethylene synthesis method
A technology of nitroethylene and synthesis method, which is applied in the chemical synthesis of 1-methylamino-1-methylthio-2-nitroethylene and the intermediate field of synthetic peptic ulcer drug ranitidine, which can solve complex problems. , product quality decline, many by-products, etc., to achieve the effect of reducing unit operations and equipment, reducing energy consumption, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The present invention will be further described below.
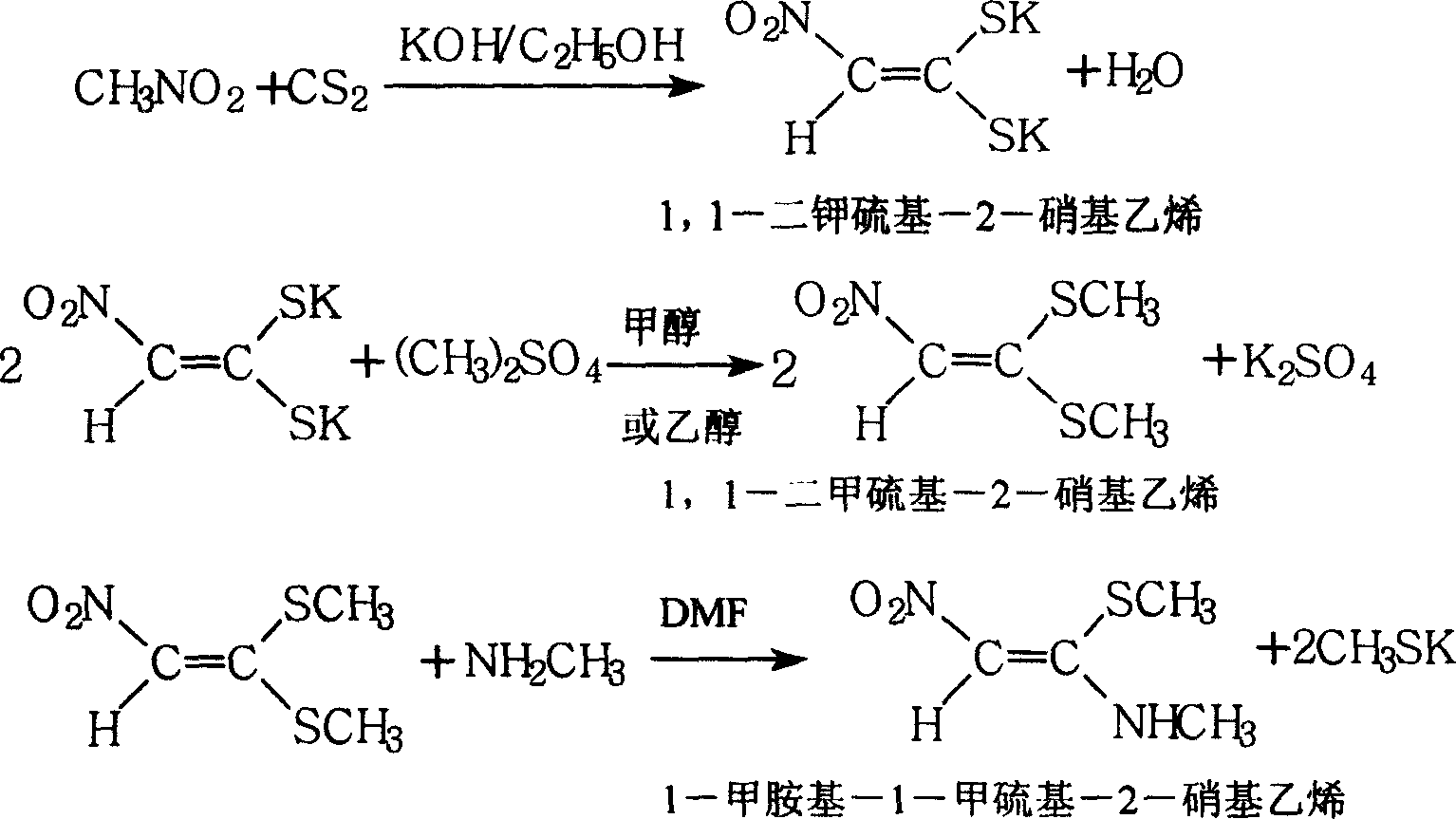
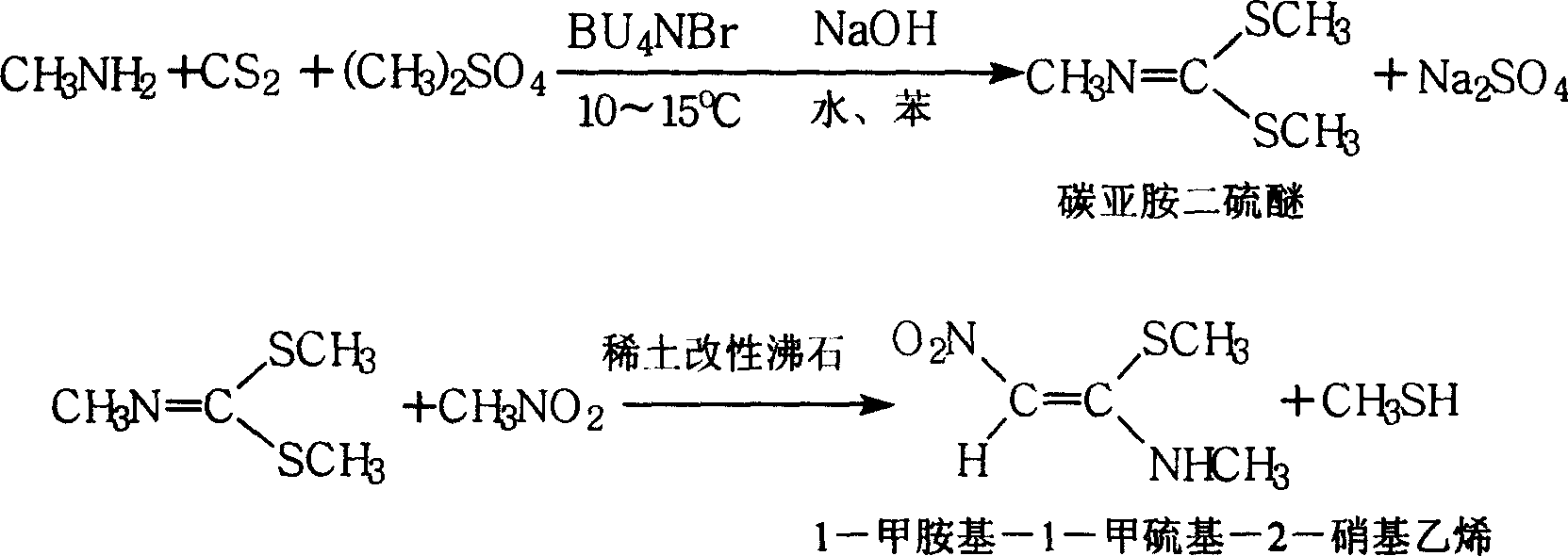
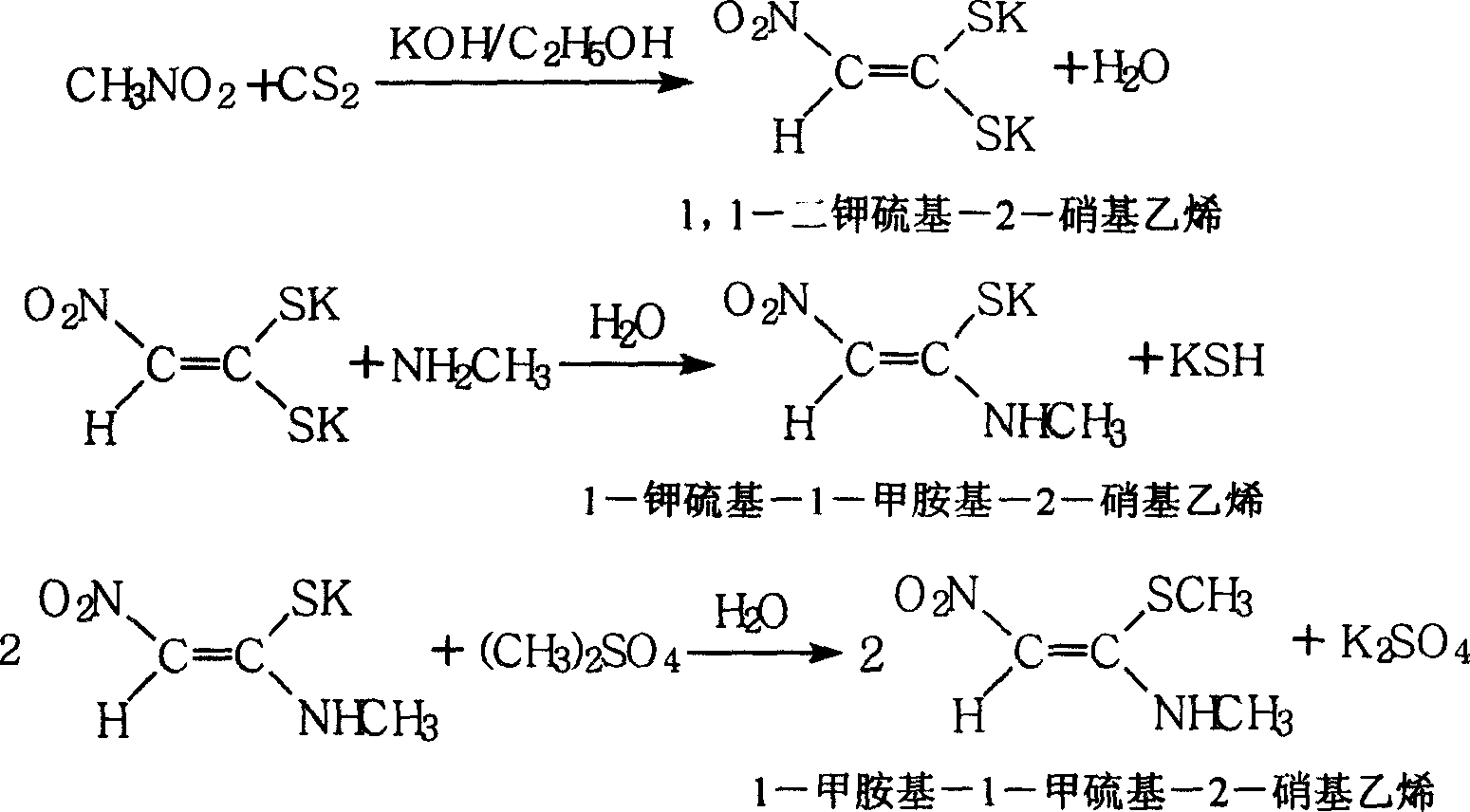
[0048] A synthetic method of 1-methylamino-1-methylthio-2-nitroethylene, comprising the following steps:
[0049] (1) Preparation of Potassium Hydroxide Ethanolic Solution
[0050] Add 190kg KOH and 400kg absolute ethanol into a 1000 liter enamel or stainless steel reaction kettle for alkali dissolution, heat to 40-80°C under stirring to dissolve, and cool down to room temperature for use after complete dissolution.
[0051] (2) Preparation of 1,1-dipotassium thio-2-nitroethylene
[0052] Add 150kg of nitromethane, 170kg of carbon disulfide and 150kg of absolute ethanol to a 2000-liter enamel or stainless steel addition reaction kettle, and start adding potassium hydroxide ethanol solution dropwise at 20°C under stirring. hours, then cooled down to below room temperature, and filtered to obtain brick red solid 1,1-dipotassium thio-2-nitroethylene, and the filtrate ethanol was recovered and recycled.
[0053] (3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com