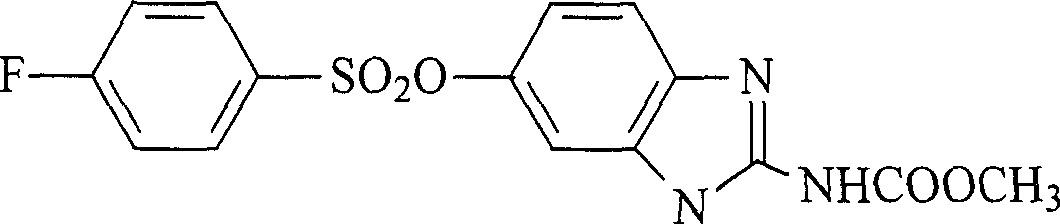
Method for preparing 5-(4- fluobenzene sulphonyloxy) benzimidazole-2-amido methyl formate
A technology of fluorobenzenesulfonyloxy and methyl carbamate, which is applied in the field of preparing 5-(4-fluorobenzenesulfonyloxy)benzimidazole-2-methyl carbamate, which can solve unfavorable industrial production and processing Problems such as cumbersome and high unit price of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
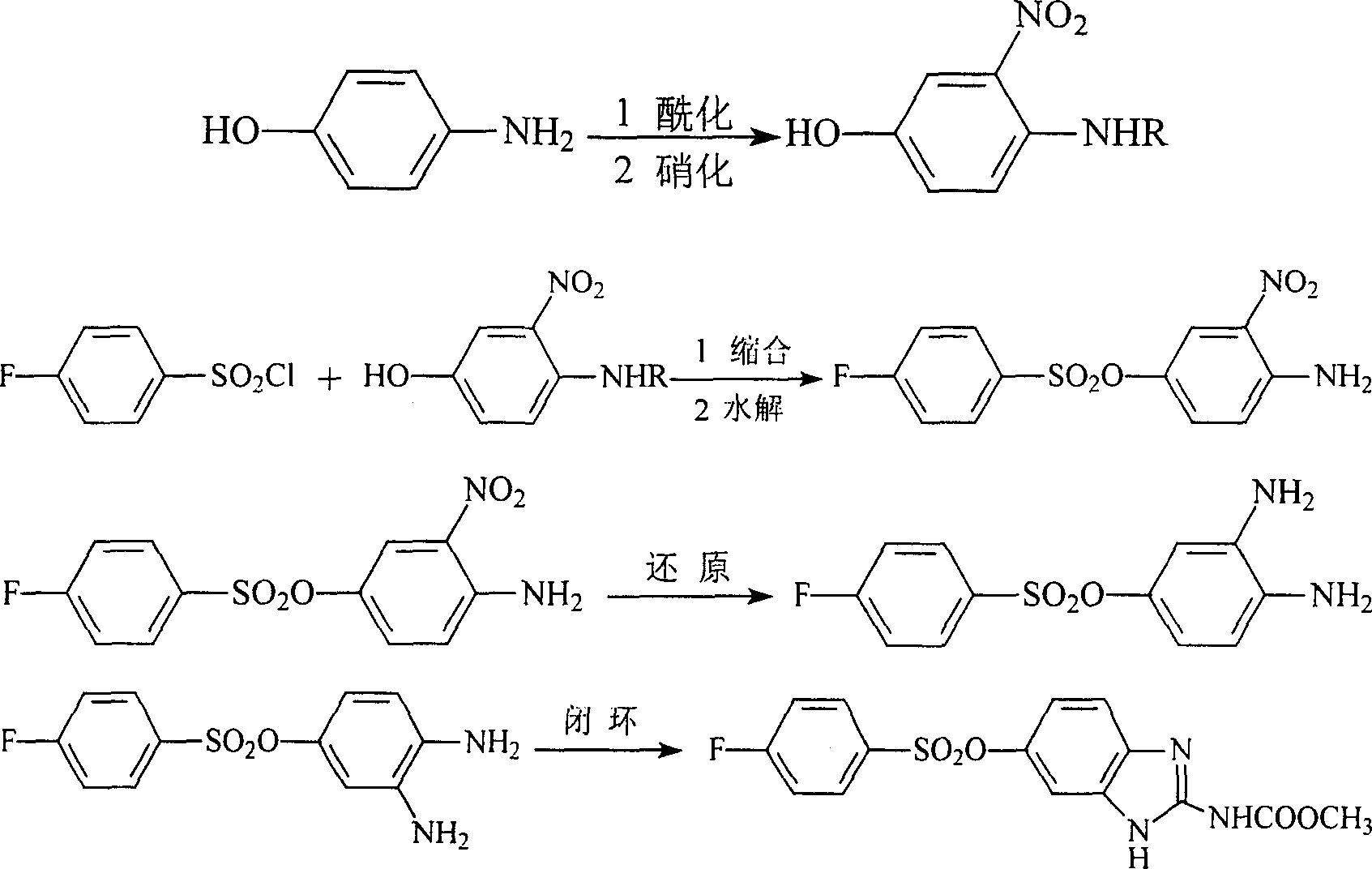
[0031] step 1
[0032] In a 150ml four-neck flask equipped with a stirring device and a thermometer, add 10.0g (0.092mol) of p-aminophenol and 10ml (0.120mol) of acetic anhydride in sequence, heat and stir, and reflux for 2 hours. The temperature continued to rise during the reaction. After the reaction was completed, the reaction solution was poured into a small amount of ice water, and crystals were precipitated. After suction filtration, the product was recrystallized with alcohol to obtain 13.2 g of paracetamol, with a yield of 94.9% and a melting point of 170°C.
[0033] step 2
[0034] In a 150ml four-necked flask equipped with a stirring device and a thermometer, add 80ml of 98% sulfuric acid, stir and cool down to below 10°C, add 9.4g (0.062mol) of paracetamol, cool to below 10°C, dropwise add 3.7ml98 % sulfuric acid and 3.8ml 96% nitric acid mixed acid solution, after adding, react for 2h. After the reaction stopped, under strong stirring, pour the reaction solutio...
Embodiment 2
[0036] Referring to the first reaction step of Example, first formylate and then nitrate to obtain 3-nitro-4-formylaminophenol.
Embodiment 3
[0038] Referring to the first reaction step of Example, propionylation and then nitration were carried out to obtain 3-nitro-4-propionylaminophenol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com